Study finds Moderna COVID vaccine efficacy peaks at 94% after 120 days
A recent research letter posted to the JAMA Network Open assessed the durability of immunity conferred by the coronavirus disease 2019 (COVID-19) messenger ribonucleic acid (mRNA)-1273 vaccine.
Background
Assessing the longevity of immunity imparted by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines is among the public health priorities. Further, the findings are required to inform guidelines on nonpharmaceutical interventions and booster vaccinations for COVID-19.
About the study
In the present work, the researchers evaluated the mRNA-1273 P301 cohort study, an ongoing phase 3, randomized, placebo-controlled trial of 30 415 US adults to assess the efficacy and safety of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine.
The team used the per-protocol population for this cohort analysis, which comprised 28,451 subjects who were SARS-CoV-2-negative at the initiation of the study and received two vaccine doses by the completion of the blinded phase. The volunteers received the initial vaccine dose between 27 July and 23 October 2020.
A minimum of two systemic symptoms or one respiratory symptom or sign defined the COVID-19 cases and were validated by a SARS-CoV-2-positive reverse transcriptase-polymerase chain reaction (RT-PCR) test result. Before enrollment, all participants submitted a written informed consent. The scientists adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting standards.
The authors used a Cox regression model that represented the log hazard ratio for the vaccine impact as a piecewise-linear function of time after immunization, with change points positioned at 120, 80, and 40 days following dose 1 at the timestamps where the log hazard ratio's slope was anticipated to change, to obtain an accurate estimation of protection. The whole curve of vaccine effectiveness (VE) on the present likelihood of disease can be estimated using this formulation.
The investigators used calendar time after the study began as the time scale for the assessment instead of time following subject randomization since the frequency of COVID-19 community transmission differed dramatically over time. This enabled them to compare the illness incidence among unvaccinated and vaccinated people on the same calendar date. The study was carried out with the version 4.1 R package DOVE, with the dove2 function.
Study findings
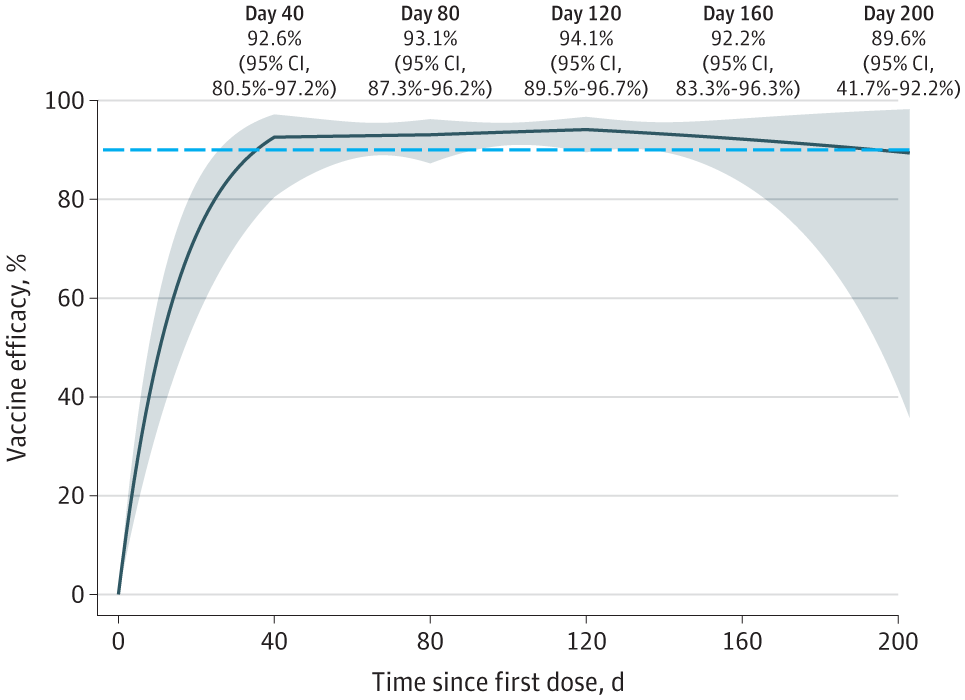
The study results indicated that the placebo group had 14,164 participants with 769 SARS-CoV-2 infection cases, while the mRNA-1273 group comprised 14,287 subjects with 56 COVID-19 incidents. The VE rose steadily to a peak of 94.1% on day 120, successive to 92.6% 40 days following the initial dose. At around 120 days, the VE began to decline, and it had fallen to 89.6% by 200 days.
Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine in Reducing the Current Risk of Symptomatic COVID-19
These findings demonstrate that VE was declining with time and was more insightful regarding the length of protection towards symptomatic illness than prior estimates. Although there was a substantial ambiguity in predicting VE close to the completion of blinded follow-up, the degree of protection was yet high 200 days following dose 1.
Conclusions
According to the study data, the COVID-19 Moderna VE against symptomatic SARS-CoV-2 infection was assessed to be 94.1% at interim screening and 93.2% at the end of the blinded phase. Upon the comparisons of these two estimations, it appears that VE was diminishing slightly.
Nevertheless, since the VE estimate was acquired under the presumption that VE was stable during the analysis period, it represents an average of the time-varying vaccine impact throughout a broad research period, weighted by when the incident happens instead of the VE after the investigation period. Hence, this comparison was insufficient to identify the actual extent of waning.
The team stated that the phase III trials offer placebo-regulated efficacy estimates for less than seven months following dose 1 due to the overlap of placebo participants with the vaccine group. Indeed, in the current investigation, few cases of COVID-19 occurred following six months, making it impossible to determine the extent of VE decline after the blinded follow-up. The authors noted that observational studies could reveal information on vaccines' long-term advantages.
- Lin D, Baden LR, El Sahly HM, et al. Durability of Protection Against Symptomatic COVID-19 Among Participants of the mRNA-1273 SARS-CoV-2 Vaccine Trial. JAMA Netw Open. 2022;5(6):e2215984, DOI:10.1001/jamanetworkopen.2022.15984, https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2793157
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Coronavirus, Coronavirus Disease COVID-19, Efficacy, Epidemiology, Frequency, immunity, Immunization, Placebo, Polymerase, Polymerase Chain Reaction, Public Health, Research, Respiratory, Reverse Transcriptase, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article