Isolating the biochemical steps that culminate in pyroptosis during CAR-T treatment

A team of researchers affiliated with several institutions in China has isolated the biochemical steps that culminate in pyroptosis during CAR-T treatment. In their paper published in the journal Science Immunology, the group describes their study of modified immune cells used in treating leukemia and what they found.
CAR-T cell therapy is used to treat patients with blood cancers—it involves removing T cells from a patient, genetically modifying them, and reintroducing them into the patient. The goal of the treatment is to give immune cells the ability to attack and kill cancer cells. But sometimes, there is a very serious side effect called cytokine release syndrome, in which the modified T cells spur overproduction of cytokine, resulting in dangerous levels of inflammation.
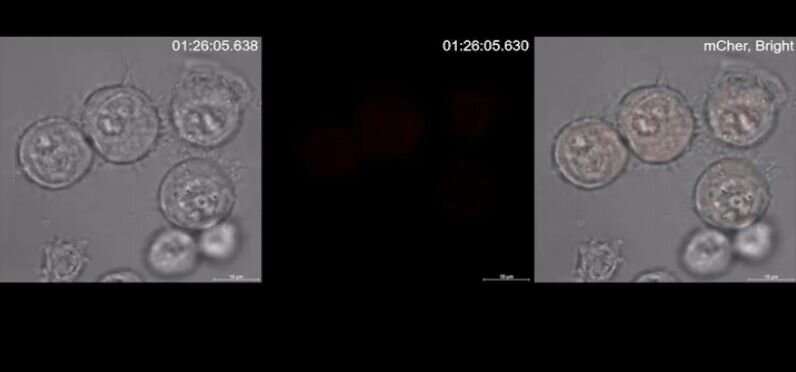
Under normal circumstances, when a cell dies naturally, it shrinks and breaks apart and is cleaned out by the immune system. With cytokine release syndrome, the cancer cells that are attacked by the genetically modified T cells experience a different kind of death called pyroptosis. They swell up and burst, releasing their contents into the body. The immune system responds by producing cytokine chemicals that activate dangerous levels of inflammation. In this new effort, the researchers have isolated the steps that culminate in pyroptosis in a single cell.
The work involved watching very closely as genetically modified T cells were introduced to cancer cells. The team observed the CAR-T cells releasing three to four times the normal amount of perforin and granzyme B. Both are proteins—perforin poked holes in tumor cell membranes, allowing granzyme B to activate a protein called gasdermin, which also pokes holes in cell membranes.
Source: Read Full Article