FDA Approves New Device for Enlarged Prostate Treatment
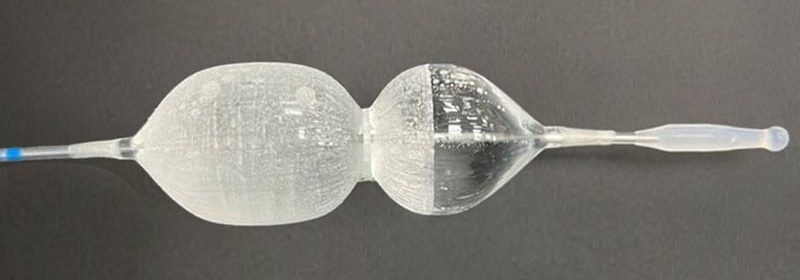
The US Food and Drug Administration has approved a new catheter designed to treat urinary tract symptoms of an enlarged prostate, also known as benign prostate hyperplasia (BPH).
Designed and marketed by Urotronic (Plymouth, Minnesota), the Optilume BPH Catheter System employs mechanical dilation to relieve obstruction of the prostate and then delivers paclitaxel to aid in prostate healing. The device is used in an outpatient setting and is less invasive than other procedures.
“There’s nothing else like Optilume BPH that’s currently available, it’s the only treatment option that requires no cutting, burning, steaming, or implants,” said Urotronic President and CEO David Perry in a press release.
Two randomized trials, EVEREST-1 and PINNACLE, both showed that the Optilume BPH system improved urinary flow rate and decreased the amount of urine stored in the bladder following urination. Men who used the OPTILUME device were able to ejaculate normally and reported no sexual difficulties.
“Optilume BPH is the next generation of minimally invasive technology, creating a new drug device space among BPH therapies,” said Steven A. Kaplan, MD, professor of urology at the Icahn School of Medicine at Mount Sinai in New York City, in the press release. Kaplan led the EVEREST-1 and PINNACLE studies, which Urotronic funded.
More than 80% of men older than age 70 have an enlarged prostate, based on autopsy analyses.
Marcus A. Banks, MA, is a journalist based in New York City who covers health news with a focus on new cancer research. His work appears in Medscape, Cancer Today, The Scientist, Gastroenterology & Endoscopy News, Slate, TCTMD, and Spectrum.
For more news, follow Medscape on Facebook, Twitter, Instagram, YouTube, and LinkedIn.
Source: Read Full Article