Study finds microbiota transfer therapy provides long term improvement in gut health in children with autism
Autism Spectrum Disorder currently affects 1 in 44 children in the U.S., according to the Centers for Disease Control and Prevention. For reasons that remain murky, these numbers appear to be trending upward as researchers and clinicians struggle to find effective treatments.
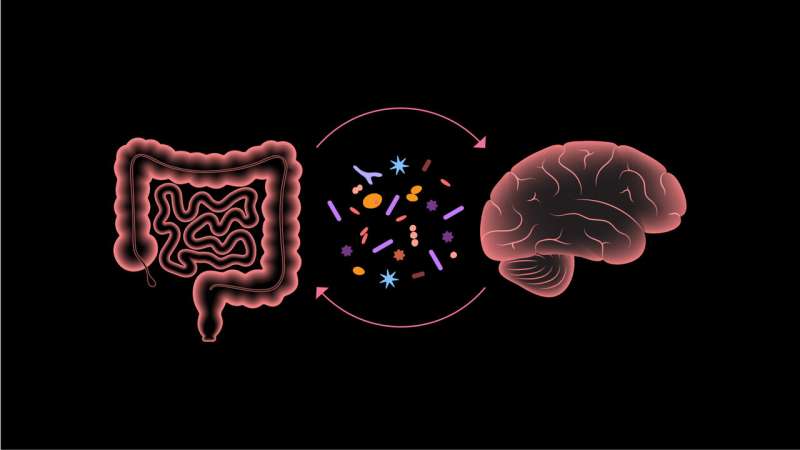
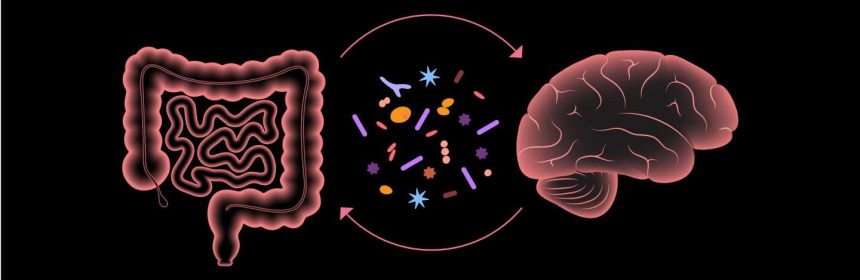
Recently, a new approach to treat symptoms associated with this disorder has emerged, thanks to the explosion of research on the trillions of non-human cells inhabiting the gastrointestinal tract—collectively known as the gut microbiome. The treatment, called microbiota transfer therapy, is a process where healthy gut bacteria are transferred to children with autism.
In a new study, Arizona State University researchers and their colleagues deeply explore changes in the gut microbiota following microbiota transfer therapy—specifically, by using whole genome sequencing, they looked at alterations in bacterial species and genes involved with microbial metabolism.
The researchers discovered that microbial taxa and genes that are important for microbial pathways associated with improvements in the physical and behavioral symptoms of autism spectrum disorder, improved following microbiota transfer therapy.
In first-of-its-kind research, the research team used a whole genome sequencing technology known as “shotgun metagenomics” to extract detailed data from more than 5,000 bacterial species found in the gut of children with autism spectrum disorder before and after microbiota transfer therapy. The researchers then compared these results with bacterial populations in the guts of healthy children.
The results showed considerable improvement in overall abundance of bacteria following the microbiota transfer therapy, and this confirmed previous findings published in Scientific Reports in 2019. Also, there were substantial increases in populations of beneficial bacterial species typically found in lower numbers in children with autism.
Additionally, two genetic indicators of dysregulation in the gut microbiome of children with autism improved following microbiota transfer therapy. These key genetic markers are the metabolism of sulfur and the failure to detoxify oxidative stress.
The findings are encouraging because the severity of gastrointestinal dysfunction in autistic children appears proportional to the degree of behavioral and cognitive issues, highlighting the importance of the gut-brain axis—a topic of intense interest in the world of microbiomics. The gut-brain axis is the communication system between your brain and your gut.
“This study highlights altered levels of important bacterial species and metabolic genes in children with autism and improvements after microbiota transfer therapy,” says Khemlal Nirmalkar, lead author and post-doctoral fellow working in the Rosa Krajmalnik-Brown lab at the ASU Biodesign Institute. “Our long-term goal is to understand the functional role of the gut microbiome, fill the knowledge gap of the gut-brain axis in autism, and identify therapeutic targets to improve GI health and behavior in children with autism.”
“Completing more in-depth microbiome sequencing is important because it can help us better understand what microbes in the gut are doing and why they are an important part of the gut-brain axis,” said Krajmalnik-Brown, who directs the newly established Biodesign Center for Health Through Microbiomes. She is also a professor with the ASU School of Sustainable Engineering and the Built Environment in the Ira A. Fulton Schools of Engineering.
Collaborators include James Adams, President’s Professor with the ASU School for Engineering of Matter, Transport and Energy, and researchers with the Rensselaer Polytechnic Institute in New York. The study appears in a special issue of the International Journal of Molecular Sciences.
The research team used shotgun metagenomics, or whole genome sequencing, to better understand the bacterial populations at the species level. They also wanted to understand bacterial genes before and after microbiota transfer therapy. The treatment not only increased the abundance of beneficial bacteria but also helped to normalize altered levels of bacterial genes, particularly those related to the synthesis of folate, oxidative stress protection and sulfur metabolism, and importantly, became similar to typically developing children.
Autism remains an enigmatic disorder, often emerging in early childhood and causing lifelong developmental disabilities that affect social skills, communication, personal relationships and self-control. So far, there is no cure for the affliction and therapies for treating associated symptoms remain limited.
The microbiota transfer procedure involves the transfer of gut microbiota from healthy donors to ASD patients over a period of seven to eight weeks. The procedure begins with a 2-week antibiotic treatment and bowel cleanse, followed by an extended transplant of fecal microbiota, applying a high initial dose followed by daily and lower maintenance doses for 7–8 weeks. This treatment was initially studied in children with autism ages 7-16 years old.
In a follow-up study, the same 18 participants were examined two years after treatment was completed. Most improvements in gastrointestinal symptoms were maintained, while autism-related symptoms continued to improve even after the end of treatment, demonstrating the long-term safety and efficacy of microbiota transfer therapy as a therapy for autism.
The treatment reduced the severity of gastrointestinal symptoms by roughly 80% and ASD symptoms by about 24% by the end of treatment. After two years, the same children showed an approximate 59% reduction in gastrointestinal symptoms and 47% reduction in ASD symptoms, compared with baseline levels established prior to treatment.
Krajmalnik-Brown and Adams are currently working on phase-2 double-blind placebo-controlled studies of microbiota transfer therapy for children and adults with autism, and they plan to verify whether these findings hold true in those two studies.
Future research will further explore the role of specific microbial species, functional gene expression and the production of a range of ASD-related metabolites before and after microbiota transfer therapy.
More information:
Khemlal Nirmalkar et al, Shotgun Metagenomics Study Suggests Alteration in Sulfur Metabolism and Oxidative Stress in Children with Autism and Improvement after Microbiota Transfer Therapy, International Journal of Molecular Sciences (2022). DOI: 10.3390/ijms232113481
Dae-Wook Kang et al, Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota, Scientific Reports (2019). DOI: 10.1038/s41598-019-42183-0
Journal information:
Scientific Reports
Source: Read Full Article