Researchers identify potential target in myeloid malignancies
Researchers at Children’s Hospital of Philadelphia (CHOP) have identified a molecule that plays a role in post-translational modification and activation in myeloid malignancies, making it a potential therapeutic target. The findings were published today in the Journal of Clinical Investigation.
A group of proteins known as RAS proteins control signaling pathways that regulate several important aspects of normal cell growth and, when dysregulated, malignant transformation. Mutations in proteins along the RAS pathway are among the most prevalent oncogenic drivers in cancers, so there has been interest among researchers in targeting this pathway to disrupt the growth of cancer cells. Mutations in the KRAS and NRAS genes are frequently identified in myeloid disorders (15%–60%), including acute myeloid leukemia (AML) and chronic myelomonocytic leukemia (CMML).
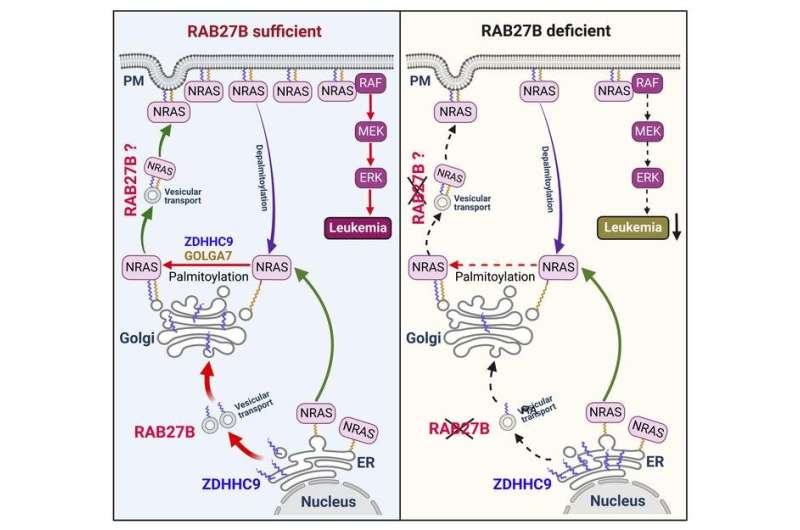
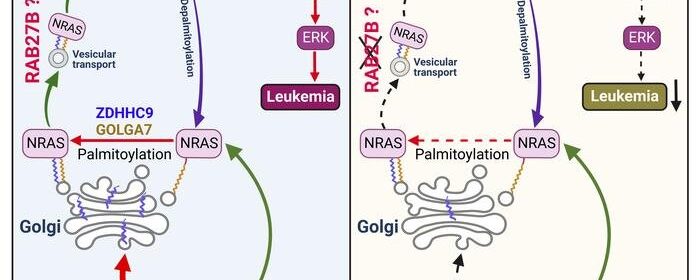
RAS proteins broadcast signals only when they are associated with cellular membranes; this happens when fat molecules, known as lipids, are added to the RAS protein, allowing it to be trafficked across cellular membranes. The cycle of trafficking across membranes is very fast, occurring every 10 to 20 minutes. The CHOP team decided to explore the potential of inhibiting this membrane trafficking by identifying targets that facilitate this process.
In doing so, the researchers discovered that an enzyme called RAB27B facilitates adding lipids to NRAS and for trafficking NRAS to the plasma membrane, which is required for activation. Through proteomic studies, the researchers discovered that RAB27B is upregulated in CBL or JAK2 mutated myeloid malignancies and that its expression correlates with poor prognosis in AML.
Further, the researchers found that RAB27B depletion inhibited the growth of CBL deficient or NRAS mutant cell lines. They also found that Rab27b deficiency in mouse models significantly reduced CMML development in vivo. Importantly, they observed that RAB27B depletion in primary human AMLs inhibited oncogenic NRAS signaling and leukemic growth. The researchers further revealed a significant correlation between RAB27B expression and sensitivity in AML to MEK inhibitors, which target mutations on the RAS pathway.
“Our study presents a link between RAB proteins and fundamental aspects of RAS posttranslational modification and trafficking, highlighting future therapeutic strategies for RAS-driven cancers,” said senior author Wei Tong, Ph.D., investigator and Professor of Pediatric Hematology at CHOP. “These findings indicate that RAB27B is a safe and promising therapeutic target for CBL or NRAS mutant malignancies.”
More information:
Jian-Gang Ren et al, RAB27B controls palmitoylation-dependent NRAS trafficking and signaling in myeloid leukemia, Journal of Clinical Investigation (2023). DOI: 10.1172/JCI165510
Journal information:
Journal of Clinical Investigation
Source: Read Full Article