Pfizer Begins Testing Its Vaccine in Young Children
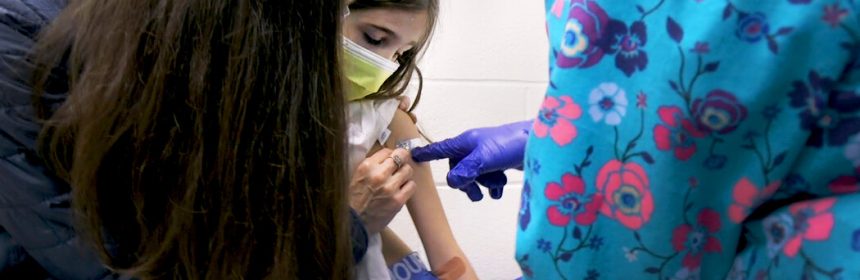
Other drug makers have begun similar trials of their Covid-19 vaccines. If they work in children younger than 12 as expected, it will be easier for the U.S. to reach herd immunity.
By Apoorva Mandavilli
Pfizer has begun testing its Covid-19 vaccine in children under 12, a significant step in turning back the pandemic. The trial’s first participants, a pair of 9-year-old twin girls, were immunized at Duke University in North Carolina on Wednesday.
Results from the trial are expected in the second half of the year, and the company hopes to vaccinate younger children early next year, said Sharon Castillo, a spokeswoman for the pharmaceutical company.
Moderna also is beginning a trial of its vaccine in children six months to 12 years of age. Both companies have been testing their vaccines in children 12 and older, and expect those results in the next few weeks.
AstraZeneca last month began testing its vaccine in children six months and older, and Johnson & Johnson has said it plans to extend trials of its vaccine to young children after assessing its performance in older children.
Immunizing children will help schools to reopen as well as help to end the pandemic, said Dr. Emily Erbelding, an infectious diseases physician at the National Institutes of Health who oversees testing of Covid-19 vaccines in special populations.
An estimated 80 percent of the population may need to be vaccinated for the United States to reach herd immunity, the threshold at which the coronavirus runs out of people to infect. Some adults may refuse to be vaccinated, and others may not produce a robust immune response.
Source: Read Full Article

