Innovative techniques reveal how scarring occurs in long COVID and pulmonary fibrosis
An international team of researchers has revealed how scarring occurs in long COVID and pulmonary fibrosis using innovative blood biomarkers and X-ray technology. This study, published in eBioMedicine, contributes to the knowledge on the pathophysiology of severe COVID-19 and thus its treatment.
Long COVID syndrome, or the origin of the long-term consequences of SARS-CoV-2 infection, is still not fully understood, more than two years after the onset of the pandemic. In particular, the long-term changes in lung tissue following severe COVID-19 disease pose significant limitations for many patients. Some of these patients continue to develop post-COVID pulmonary fibrosis, which is characterized by rapid scarring of the lung tissue.
Until now, the scientific community didn’t understand the underlying mechanisms of this scarring and of specific blood markers that can predict this process.
Now, an international research team led by doctors and researchers at the Institute of Pathology at the RWTH Aachen University Hospital, the Hannover Medical School (MHH), HELIOS University Hospital in Wuppertal, and the University Medical Center Mainz, in collaboration with scientists at University College London (UCL) and the European Synchrotron (ESRF), has uncovered the mechanism that modifies the connective tissue of the lung in severe COVID-19.
By combining the latest in imaging and molecular biology techniques this multidisciplinary team uncovered a mechanism by which the connective tissue of the lung is modified in severe COVID-19. They have demonstrated how COVID-19 changes the structure of the finest blood vessels in the lung and found molecular markers of this damage in the blood of patients that might ultimately help diagnose and treat the condition.
Pulmonary fibrosis and COVID-19
The term “pulmonary fibrosis” covers a variety of different lung diseases in which persistent inflammation leads to progressive scarring of the lung scaffold. Although these serious diseases can be mitigated with medication, they are still incurable and usually have higher mortality rate than many cancers.
For many of those affected, lung transplantation is the only remaining, life-saving therapy. Epidemiological data from patients with severe COVID-19 suggest that approximately 20% of hospitalized patients develop post-COVID pulmonary fibrosis, which varies greatly in its extent and progression and can only be predicted very imprecisely by routine clinical imaging.
HiP-CT unveils damage in lungs
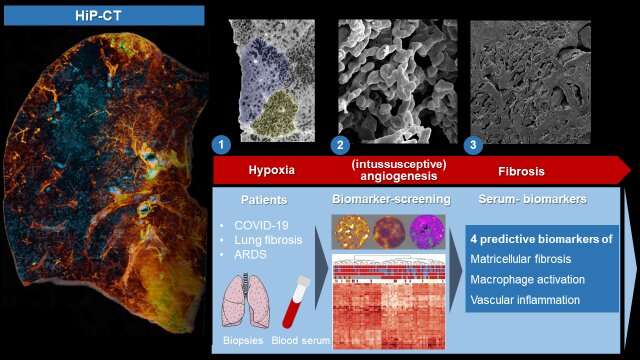
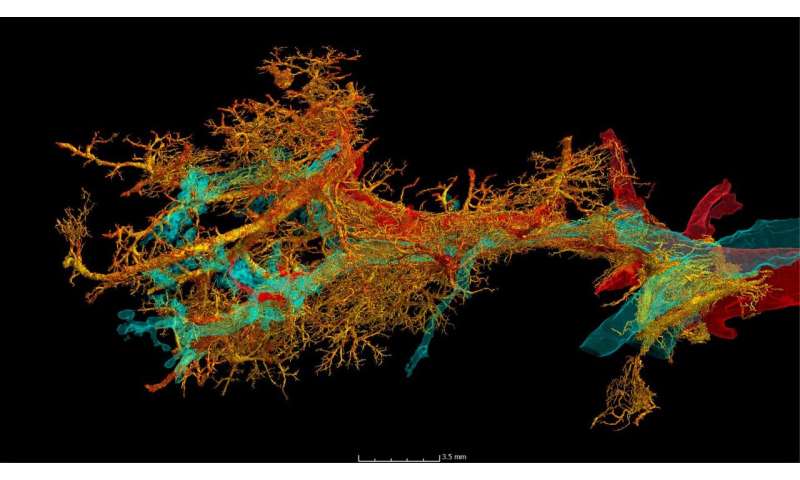
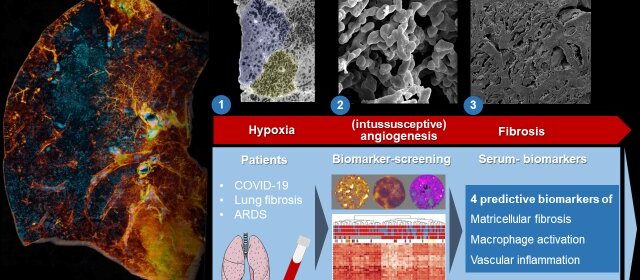
To reveal the microscopic tissue alterations in this scarring process in detail for the first time, the researchers examined intact lungs of severely diseased COVID-19 using the latest 3-D imaging technique, Hierarchical Phase-Contrast Tomography or HiP-CT, developed at the world’s brightest synchrotron X-ray source, the ESRF—European Synchrotron, in France.
The researchers then compared the imaging results obtained thanks to HiP-CT to the ones in the clinic and demonstrated that, in comparison to other fibrotic lung diseases and to influenza A, changes in COVID-19 are driven by micro clots (medically termed secondary lobular microischemia) and new blood vessels formation via a specific mechanism, called intussusceptive angiogenesis.
This blood vessel formation, which is typical of COVID-19, increased significantly over the course of the COVID-19 disease. Danny Jonigk, thoracic pathologist from the Institute of Pathology at the RWTH Aachen University Hospital explains that “these heterogeneously distributed, distinct changes at the level of the finest lung lobules cannot be detected via clinical imaging due to the lack of detail in current clinical technology.”
“With the new technology of HiP-CT, we were able to see, for the first time, that the scarring processes in post-COVID fibrosis are the result of generalized vascular damage caused by the SARS-CoV-2 virus.”
First author Maximilian Ackermann from Mainz and Wuppertal points out that “such results suggest that treatments aiming at preventing microvascular thrombosis and tissue ischemia (such as early oxygen supplementation and anti-coagulation) may be beneficial in patients with severe COVID-19 and avoid subsequent fibrotic remodeling.”
HiP-CT is a technique developed jointly by a UCL-ESRF team, led by UCL’s Prof. Peter Lee. “The ESRF is unique among synchrotrons in being able to do HiP-CT, both because of its Extremely Brilliant Source (EBS) upgrade and the many unique qualities of beamline BM18,” explains Lee.
Using HiP-CT, the team at ESRF, led by Paul Tafforeau, showed, for the first time, that in severe COVID- 19 there is a mosaic-like pattern to the damage in the lungs.
Claire Walsh, researcher at UCL, says that “understanding how patterns of a disease are different across a whole organ, like the mosaic-pattern, helps us to decipher the mechanisms by which the damage is happening. A technique like HiP-CT is a real game-changer for unveiling not only the really fine detail of the tissue damage, but also how it is distributed across a whole organ, and relating that back to what is seen in the clinic.”
The team also used Diamond Light Source, the UK’s synchrotron light source, to image a smaller section of lung, which showed how a reduced supply of blood to the lung tissue is due to changes in the structure of the finest blood vessels supplying the lungs.
Tissue and blood biomarkers for early fibrosis detection
The team also identified new tissue markers of severe COVID-19 and interstitial pulmonary disease. “We noticed a significant increase in inflammatory markers and markers of new blood vessel formation, which are responsible for the clinical deterioration of affected patients,” says Jan-Christopher Kamp, from the Hanover-based Lung Research Group. “It is precisely the correlation of the molecular data with the morphological pattern of damage that is crucial.”
It was particularly important for the international research team not only to investigate tissue markers of severe COVID-19 disease, but also to identify plasma markers of early fibrotic remodeling that could serve as predictors of disease severity and therapeutic response in COVID-19.
They were able to identify three “key markers”, secreted into the lung tissue, in spaces between cells. By comparing blood and tissue biomarkers in COVID-19 and in patients with other forms of pulmonary fibrosis, such as idiopathic pulmonary fibrosis (IPF), the team could correlate early morphological and molecular features of the development of pulmonary fibrosis with increased levels of the involved proteins in the blood.
“These ‘predictive serum biomarkers’ enable us to find new therapeutic approaches and to start therapy for the affected patients as early as possible, when the scarring processes can still be reversed via drug therapy,” explains Detlef Schuppan from the University Medical Center Mainz and Harvard Medical School.
More information:
Maximilian Ackermann et al, The fatal trajectory of pulmonary COVID-19 is driven by lobular ischemia and fibrotic remodelling, eBioMedicine (2022). DOI: 10.1016/j.ebiom.2022.104296
Journal information:
EBioMedicine
Source: Read Full Article