Antibody validation and rapid peptide mapping technology
 Thought LeadersBin MaCo-Founder and PresidentRapid Novor, Inc
Thought LeadersBin MaCo-Founder and PresidentRapid Novor, IncTo begin, can you give our readers a brief introduction into antibody validation – what is it, why is it used, and what does it involve?
Antibodies’ intrinsic ability to bind antigens is widely exploited in the life sciences, particularly as detection tools for target proteins in research. To ensure the correctness of the detection, antibodies must be validated to demonstrate they are specific, selective, and reproducible in the same context of their intended use.
Traditional validation involves testing the interactions between the antibodies and their binding partners through controlled experiments. Such function-based validation ensures the purchased or produced antibodies have the desired properties. However, it does not guarantee that the antibody being tested is exactly the same as the one received in an earlier batch or that it has the same sequence as the one intended to be produced.
What is the difference between antibody validation testing for specificity and antibody validation testing for reproducibility?
A specificity test ensures that the antibody binds only to the target it’s supposed to bind. A reproducibility test mostly involves testing the same antibody over time with different lots to ensure each antibody always gives the same result.
Why is the verification of protein sequences crucial for antibody validation?
Firstly, batch-to-batch variability is a huge problem in the research antibody market. There have been cases where people have trusted a kit, and they see a positive reaction only to, unfortunately, realize that the kit was actually detecting another protein because the kit’s antibody batch had changed.
A certificate proving that the protein sequence of an antibody remains unchanged would have easily avoided this mishap. Secondly, more and more antibodies today are produced recombinantly from a known amino acid sequence. One needs to make sure that the end product, which is the protein produced by the expression system, indeed has the same exact sequence.
In recent years, how has peptide mapping evolved?
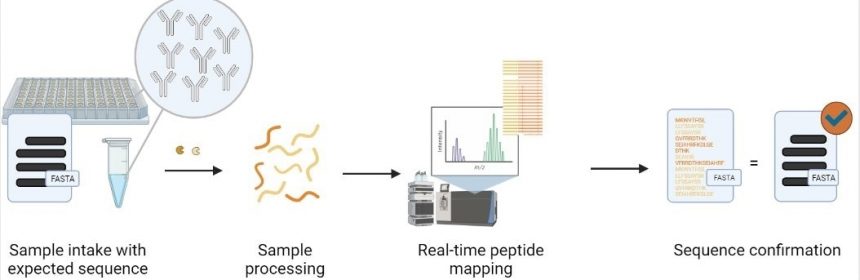
Peptide mapping involves the enzymatic digestion of the protein and the separation and detection or identification of each resulting peptide. The separation is usually done with liquid chromatography (LC). The detection or identification can be done either with a UV detector or via mass spectrometry (MS) or tandem mass spectrometry (MS/MS). More recent efforts have also begun to use multiple enzymes to digest the protein to increase the sequence coverage.
Can you tell us about your rapid peptide mapping technology, MATCHmAbTM?
Our MATCHmAbTM service is designed for antibody sequence verification. To use the service, a customer sends their antibody sample and the amino acid sequence of the antibody. We digest the antibody with one or two enzymes in our lab and analyze the resulting peptides with LC-MS/MS and our proprietary peptide identification software.
If the antibody’s sequence is correct, the analysis can usually lead to a 100% or near 100% sequence coverage. This means that each amino acid of the sequence is covered by at least one peptide identified from the mass spectrometry data. And vice versa, a significant lower sequence coverage usually indicates a sequence error.
What was the main inspiration behind MATCHmAbTM?
The reproducibility crisis caused by batch-to-batch variation is a huge problem in the antibody industry. Scientists often rely on our services to overcome reproducibility-related bad experiences (e.g., major loss of money and time). Many of our own scientists also have molecular biology and biochemistry backgrounds where they relied on antibodies, and they too are well aware of the inconsistency in antibody batches.
As its amino acid sequence uniquely determines an antibody, we believe the most assuring way to address this problem is to ensure that the antibody in each batch has exactly the same sequence. Many of our clients already routinely ensure sequence consistency by de novo sequencing the antibody with our REmAb® service and comparing the sequence of each batch. However, we would like to offer a cheaper and faster solution when the client already knows the sequence (either because the antibody is produced recombinantly from a known sequence or the antibody in another batch has already been de novo sequenced). That is why we started MATCHmAb™.
During the development of MATCHmAbTM, what were some of the most significant obstacles you came up against, and how were these overcome?
Fortunately, we had previously laid the groundwork needed to develop MATCHmAb™ as our REmAb® platform has de novo sequenced thousands of antibodies. Our main development effort on MATCHmAb™ was to optimize for cost and turnaround time. This involved many choices, from determining the best combination of enzymes to fine-tuning our internal sample processing workflow. Automation was also key in driving down the cost and turnaround time. We strived to make MATCHmAb™ an affordable and rapid solution for every researcher to validate every antibody batch.
How does MATCHmAbTM differ from other peptide mapping technology?
In terms of MS-based peptide mapping, most labs perform similar experiments. The main difference of MATCHmAb™ is that it uses the same lab and analytical software as our REmAb® de novo sequencing service. The performance of both the lab and the software has been tested and continuously optimized through de novo sequencing of several thousand antibodies in the past. This ensures that more peptides can be mapped correctly, and the clients get the most coverage of their antibodies.
In addition, if MATCHmAb™ discovers that the antibody may be different from the given sequence, a client can optionally upgrade to the REmAb® service to obtain the antibody’s full sequence. This way, the client is not limited by only knowing the new antibody is different but can also secure the new antibody’s exact sequence.
What are the benefits/advantages of rapid peptide mapping?
With MATCHmAb™, clients have been able to reduce the risk of failure, in addition to saving on both cost and time from possible downstream issues. What’s most attractive about our rapid peptide mapping service is its great reproducibility check while the client conducts specificity experiments.
Furthermore, in contrast to other peptide mapping services, it’s an easy-to-use service to check on many routinely relied-on antibodies for an affordable price. It ensures that internal processes and pipelines remain robust. It’s peace of mind by knowing that the antibody used is the same molecule intended for use.
What are some of MATCHmAbTM applications – who can use it?
MATCHmAb™ can be used by any scientist who routinely relies on immunoreagents or who develops therapeutics for their work. Perhaps even more relevant, MATCHmAbTM is a great complementary tool that antibody producers can add to their validation toolbox: routine checks on established hybridoma or recombinant cell lines, checkpoints during the development of antibody producing cell lines, or for IP protection.
MATCHmAbTM could very well be the first check-in troubleshooting binding issues, saving manufacturers both time and money and ensuring assay reproducibility across experiments for their clients. In this way, MATCHmAbTM goes beyond verifying a sequence: it secures trust. But as mentioned, this is really an invaluable weapon scientists can add to their arsenal for validation. For example, researchers working on antibody engineering could rapidly verify that their expressed protein matches the engineered sequence while gaining invaluable information on PTMs and insight that could justify further fine-tuning. The latter would be particularly useful for biosimilar development.
Where can readers find more information?
Readers can find more information through our resources on antibody validation, why the protein sequence is important for antibody validation, and through our MATCHmAbTM peptide mapping and analysis service page.
About Rapid Novor
Rapid Novor is the world leader in next-generation protein sequencing (NGPS). Through breakthroughs in proteomics and bioinformatics, the Rapid Novor team routinely helps biotech and pharmaceutical companies mine the full potential of the humoral response for immunoreagent, diagnostics, and/or therapeutic development.
Thanks to Rapid Novor’s NGPS-powered antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and implemented the first recombinant polyclonal antibody diagnostics.
Our technology has empowered thousands of researchers with the potential to unlock a breadth of discoveries to shape future research and benefit global health. At Rapid Novor, we believe that the journey to a world of more effective treatments that cost less and are easier to develop starts with decoding immunity.
About Dr. Bin Ma
Dr. Bin Ma has conducted bioinformatics research and technology commercialization for more than 20 years. Through his breakthroughs in MS-based protein sequencing technology, Dr. Ma seeks to provide new solutions to help mankind fight diseases.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Source: Read Full Article
.jpg)
