A genetically encoded sensor to measure serotonin dynamics in vivo
Serotonin (5-HT), one of the primary hormones released in the human brain, has many essential functions. For instance, serotonin is known to stabilize the mood, produce feelings of well-being, regulate sleep patterns and facilitate communication between cells.
As serotonergic signaling in the brain is known to play a crucial role in a wide range of physiological and psychological processes, drugs that increase or stabilize serotonergic activity, such as selective serotonin reuptake inhibitors (SSRIs), are commonly used to treat many psychiatric and physiological disorders.
While the functions of serotonin have been widely investigated in the past, the current understanding of cell-specific 5-HT signaling associated with different behaviors is still somewhat limited. This is in part due to the lack of reliable methods to measure serotonin levels in vivo (i.e., within living organisms).
Researchers at Peking University, University of Virginia School of Medicine and other institutes in China and the U.S. recently developed a sophisticated, genetically encoded sensor that can be used to measure and monitor serotonin dynamics. This sensor, presented in a paper published in Nature Neuroscience, could aid research focusing on serotonergic signaling, while also allowing physicians to measure serotonin levels in their patients’ brain.
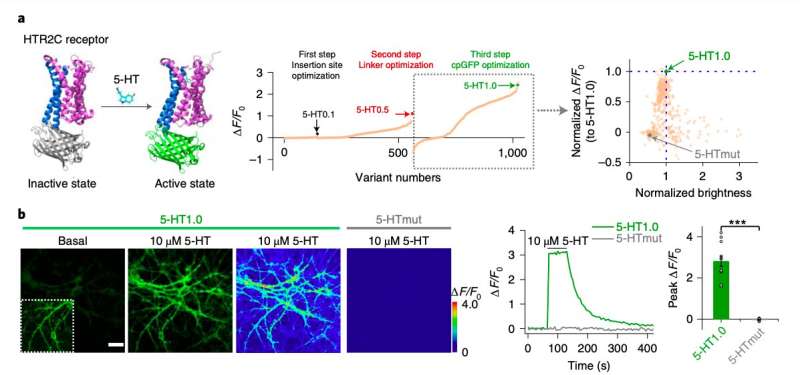
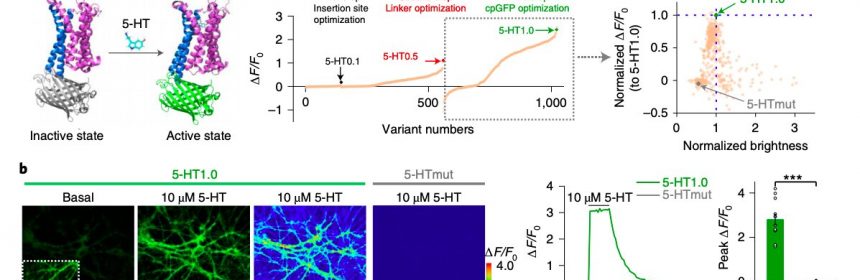
“Serotonin (5-HT) is a phylogenetically conserved monoamine neurotransmitter modulating important processes in the brain,” the researchers wrote in their paper. “To directly visualize the release of 5-HT, we developed a genetically encoded G-protein-coupled receptor (GPCR)-activation-based 5-HT (GRAB5-HT) sensor with high sensitivity, high selectivity, subsecond kinetics and subcellular resolution.”
In the past, both this research group and other neuroscientists worldwide tried to develop sensors that can detect different neurotransmitters in the brain. Many of these devices are GPCR-activation-based (GRAB) sensors, a promising class of sensors that can detect neuromodulatory signals with a high resolution.
To evaluate the performance and effectiveness of their sensor, the researchers initially tested it on cultured cells. Subsequently, they used it to detect the release of serotonin in mouse brain slices and in common fruit flies (Drosophila).
“GRAB5-HT detects 5-HT release in multiple physiological and pathological conditions in both flies and mice and provides new insights into the dynamics and mechanisms of 5-HT signaling,” the researchers wrote in their paper.
Using the sensor that they developed, the researchers were able to monitor 5-HT dynamics in living mice under specific physiological conditions, such as during sleep-wake cycles. This allowed them to confirm previous study findings and gain new insight about the ways in which serotonin helps to regulate sleep in mice and potentially other animals.
Finally, the researchers also tried to determine whether their sensor could detect the action of psychostimulant drugs. To do this, they administered methylenedioxymethamphetamine (MDMA), a drug that increases serotonin levels in the brain, to living mice, and then used their sensor to detect changes in serotonin.
“Intraperitoneal injection of MDMA caused a gradual increase in 5-HT1.0 fluorescence, which peaked after 1h and then gradually decayed over the following 3h,” the researchers explained in their paper. “This time course is comparable with that of the psychostimulation effect of MDMA in both humans and mice.”
Source: Read Full Article