New study links changes in brain immune cells to Alzheimers
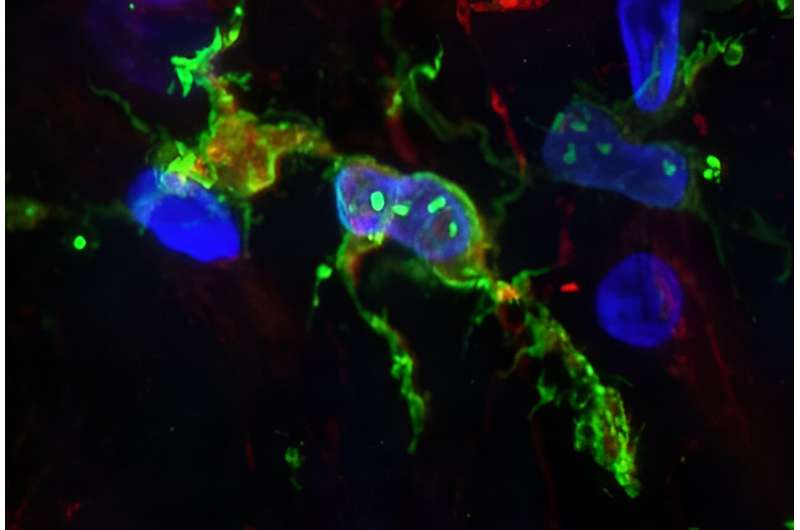
Immune cells in the brains of people who had Alzheimer’s disease appear to behave differently than those who had healthy brains for their age, according to an analysis of the cells’ gene activity.
The finding suggests it might be possible to treat Alzheimer’s disease by altering the behavior of these cells, said Katherine Prater, an expert in neuroinflammation and an acting instructor in neurology at the University of Washington School of Medicine.
“If we can determine what they are doing, we might be able to change their behavior with treatments that might prevent or slow this disease,” she said.
Prater and collaborators at the UW Medicine, Fred Hutchinson Cancer Center, Arizona State University and University of North Carolina at Chapel Hill reported their findings May 29 in the journal Nature Aging.
In the study, the researchers looked at immune cells called microglia, which play a variety of essential roles in the brain:
- They promote brain development and learning by stimulating new connections between brain cells and pruning connections we no longer need.
- They perform routine housekeeping chores such as clearing away dead cells and unwanted debris.
- They protect the brain from infection by engulfing microbes and releasing chemical signals that help orchestrate the body’s immune response.
Their role in Alzheimer’s disease, however, is less clear. For example, they appear to protect the brain by clearing out amyloid deposits, the toxic protein clumps that form in the brains of people with the disease, but they may also contribute to an inflammatory process seen in Alzheimer’s that leads to the death of brain cells.
To better understand what these cells do, the researchers examined which genes were active in microglia taken from the brains of people who had Alzheimer’s and from those who did not. Because genes control a cell’s behavior, knowing which ones are active can help reveal what a cell is likely doing.
To identify which genes were active, the scientists took advantage of the fact that when a gene is activated, the instructions encoded in its DNA sequence are copied, or transcribed, into a related molecule called RNA. Therefore, by sequencing the RNA in a cell’s nucleus, using a technique called single-nucleus RNA sequencing, it is possible to know which genes are active in different cells.
In their study, the researchers studied microglia from the brains of 12 people who died with Alzheimer’s and 10 who did not have this disease. They found that populations of microglia in both sets of brains were diverse, with the populations falling into 10 subpopulations, each of which—-based on gene activity—likely exhibit different characteristics and behaviors.
Although the microglia populations were similar in both sets of brains, the mix was different, with some populations being more prevalent in the brains affected by Alzheimer’s.
The differences could be attributed to the cells’ contributing to the destruction of brain cells seen with Alzheimer’s, or they could result from the destruction caused by the disease, Prater said.
“At this point, we can’t say whether the microglia are causing the pathology or whether the pathology is causing these microglia to alter their behavior,” she said.
Among the populations that were more prevalent in the brains affected by Alzheimer’s were cells that appear to be in a pre-inflammatory state. Those cells may have an impaired ability to perform the housecleaning tasks microglia typically do. There were also fewer protective cells that are thought to promote healthy aging.
“Now that we have determined the genetic profiles of these microglia, we can try to find out exactly what they are doing and hopefully identify ways to change their behaviors that may be contributing to Alzheimer’s disease,” Prater said.
The authors expressed gratitude to the brain donors and their families for contributing to this research. The authors also noted contributions from UW Precision Neuropathology Core and Aimee Schantz and Erica Melief from the Keene Lab in Laboratory Medicine and Pathology at UW Medicine.
This work was facilitated by the advanced computational, storage and networking infrastructure provided by the Hyak supercomputer system at the University of Washington and the Hyak team.
More information:
Katherine E. Prater et al, Human microglia show unique transcriptional changes in Alzheimer’s disease, Nature Aging (2023). DOI: 10.1038/s43587-023-00424-y
Journal information:
Nature Aging
Source: Read Full Article