Mast cells shown to have an important impact on the development of chronic myeloid leukemia
Chronic myeloid leukemia (CML) is a type of blood cancer that arises from malignant changes in blood-forming cells of the bone marrow. It mainly occurs in older individuals and represents about 20% of all adult leukemia cases.
A research team led by Dr. Sebastian Halbach, Melanie Langhammer and Dr. Julia Schöpf from the Institute of Molecular Medicine and Cell Research at the University of Freiburg has now demonstrated for the first time that mast cells play a crucial role in the development of CML. Mast cells could therefore serve as an additional therapeutic target in the clinic. “It was really impressive to see that mice lacking mast cells no longer developed severe CML,” says study leader Halbach. The results were recently published in the journal Leukemia.
Significantly elevated cytokine levels
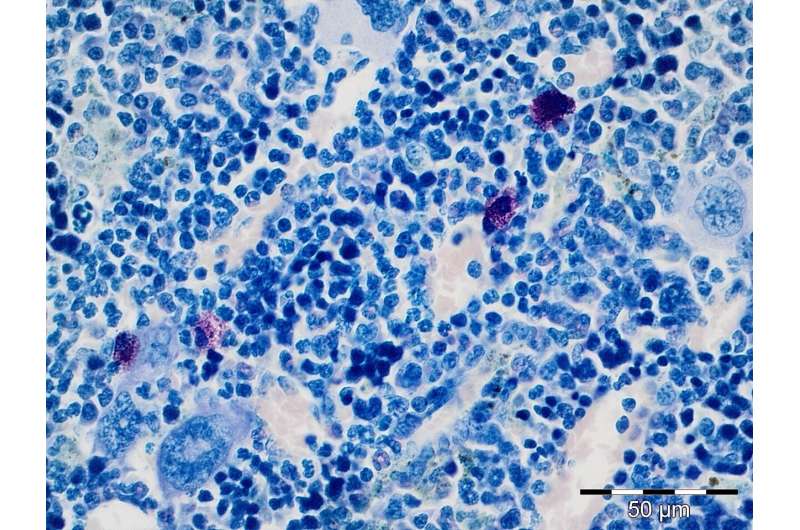
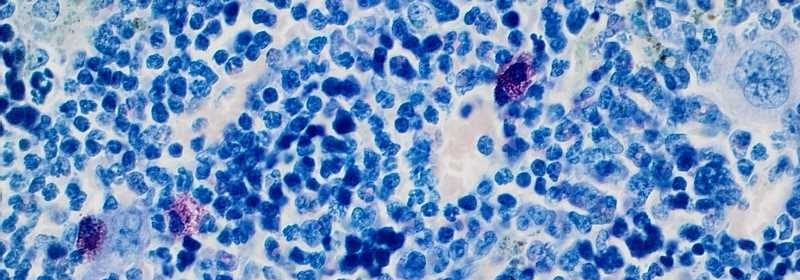
Mast cells are cells of the immune system that play a decisive role in the defense against pathogens, but also in allergies. In this context, mast cells release inflammation inducing messenger molecules, so-called proinflammatory cytokines, which are crucial for the immune response. However, proinflammatory cytokines are also frequently found in the microenvironment of tumors and are suspected of decisively promoting cancer development. Using a mouse model for CML, the scientists were able to demonstrate for the first time that cytokines in CML could indeed originate from mast cells.
First, the researchers found an unusually high number of mast cells in the bone marrow of mice showing leukemia symptoms. In subsequent experiments, they were able to demonstrate that the oncogene Bcr-Abl, as the cancer-causing protein in CML, had taken control of these mast cells. This resulted in a significantly increased release of proinflammatory cytokines. Consequently, mice lacking mast cells due to their genetic predisposition did not show an increase in proinflammatory cytokines. Moreover, these animals did not develop splenomegaly, a pathological enlargement of the spleen frequently observed in leukemias.
Clinical data support findings
For the study, the team collaborated with Prof. Dr. Tilman Brummer, Professor for Signal Transduction and Medical Cell Research at the University of Freiburg, Dr. Khalid Shoumariyeh and Prof. Dr. Heiko Becker from the University Medical Center Freiburg, and Dr. Mirle Schemionek-Reinders and Prof. Dr. Michael Huber from the University Medical Center Aachen. With the help of the partners, the findings from the animal model could finally be supported by clinical data from CML patients: On the one hand, it was shown that patients with severe splenomegaly often have an increased number of mast cells in their bone marrow. On the other hand, patients with increased concentrations of tryptase, a lead enzyme of mast cells, also had increased levels of proinflammatory cytokines in their blood.
“These results could be the basis for new therapeutic approaches,” Halbach explains. The discovery of the Bcr-Abl oncogene as the trigger for CML has made it possible to develop so-called tyrosine kinase inhibitors (TKIs), which revolutionized the therapy. However, it is often not possible to eliminate all malignant cells with these drugs, especially the leukemia stem cells in the bone marrow, which is why lifelong treatment is necessary. In addition, resistances to the TKIs can develop during therapy, leading to relapse. Moreover, a lifelong use of TKIs is associated with a high burden of side effects for patients.
“It is therefore of great importance to develop new and more effective therapies,” says Halbach. And the study also offers suggestions for further research into many types of cancer beyond CML. “I am convinced that mast cells also play an important role in other cancers, since proinflammatory cytokines are often found upregulated here as well.”
More information:
Melanie Langhammer et al, Mast cell deficiency prevents BCR::ABL1 induced splenomegaly and cytokine elevation in a CML mouse model, Leukemia (2023). DOI: 10.1038/s41375-023-01916-x
Journal information:
Leukemia
Source: Read Full Article