How inflammation in COVID-19 alters the smallest vessels in the heart
Severe courses of COVID-19 infection not only impair lung function, but can also cause life-threatening consequences for the heart. The spectrum ranges from acute inflammation of the heart muscle (myocarditis) to chronic restriction of the heart’s pumping function. The basic patterns of damage have not yet been completely proven.
An interdisciplinary research team led by Professor Dr. Danny Jonigk, Christopher Werlein and Private Lecturer (PD) Dr. Mark Kühnel from the Institute of Pathology at the Hannover Medical School (MHH) has now used innovative molecular methods and a high-resolution microscopy technique to show how the ongoing inflammation in COVID-19 attacks the heart tissue and, in the long term, remodels the smallest coronary vessels by directing special precursor cells of the immune system from the blood into the heart. The study was published in the journal Angiogenesis.
False-activated inflammatory cells
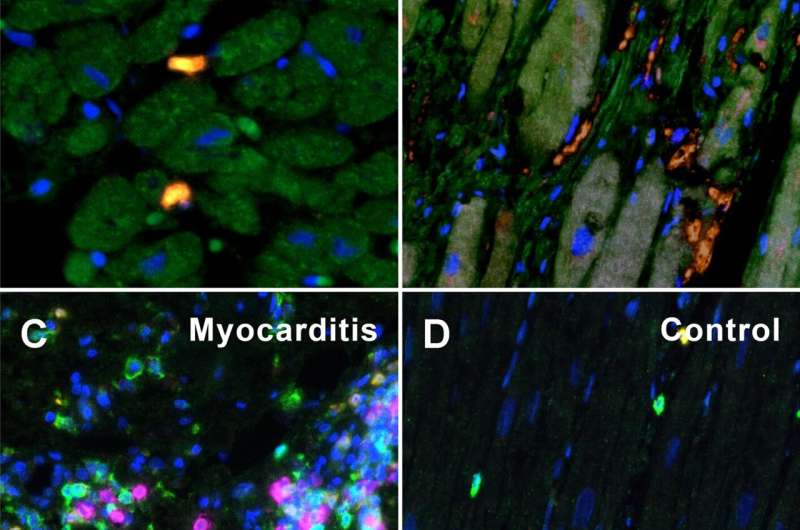
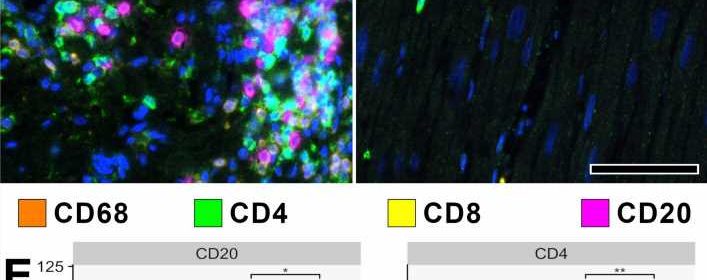
About one in three people complain of discomfort and functional impairment of the heart after severe COVID-19 disease. In order to elucidate the mechanisms of this long-lasting myocardial damage, the researchers examined heart tissue from patients with severe COVID 19 and compared it with tissue samples taken after severe influenza infections caused by the influenza virus and after severe myocarditis caused by other viruses.
Although in the COVID 19 heart injury—unlike the comparison samples—there was no classic inflammation of the heart tissue on the outside, the research team did find a large accumulation of false-activated inflammatory cells: so-called macrophages and their precursor cells, the monocytes. “These monocytes have an outstanding importance as precursor cells of new blood vessel formation and can remodel the blood vessel system in a very short time,” explains Christopher Werlein, first author of the study.
COVID-19 attacks all vessels in the body
Triggered by the infection with the coronavirus SARS-CoV-2, tiny blockages accumulate in the heart vessels, which are only a few millimeters thick. “These ultra-thrombi change the blood flow considerably and thus also the oxygen supply,” emphasizes PD Dr. Kühnel. This calls the monocytes into action, which attach themselves to the inner vessel walls and form new branches there.
This vascular remodeling—known as intussusceptive angiogenesis—has already been described by the team as a characteristic pattern of damage in other organs of COVID 19 patients. What may be intended as a short-term rescue response by the body to compensate for reduced blood flow and undersupply of oxygen could lead to chronic damage to the heart and long COVID, the researchers suspect.
Source: Read Full Article