Study reveals hidden inactive form of p38a protein
 Reviewed
Reviewedp38a protein, a key enzyme in the regulation of various cellular functions, plays a crucial role in some diseases, including cancer, chronic inflammation, and neurodegenerative conditions. Since the discovery of p38a, various pharmaceutical companies and numerous research groups have dedicated considerable efforts to develop inhibitors of this protein. However, the results have not met the expectations foreseen to be able to design drugs.
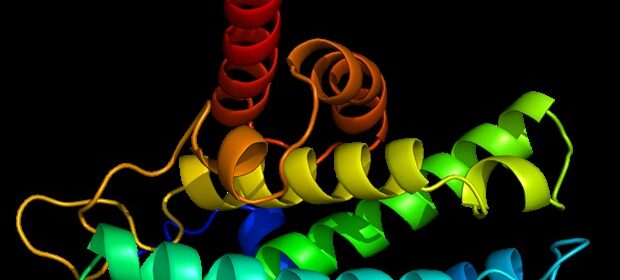
A team of researchers led by Dr. Maria Macias and Dr. Angel R. Nebreda, both ICREA researchers at IRB Barcelona, has discovered that p38a adopts a conformation not previously described. In brief, they have revealed a new "oxidized" form, in which a disulfide bridge is established. The protein would adopt this form temporarily depending on the redox state of the cell. This new form of p38a, which has been described in the journal Nature Communications, does not allow binding with activators or substrates and it is therefore unable to perform its characteristic functions. However, this process is reversible, and protein function is recovered under reducing conditions.
The identification of a new form of p38a could explain previous difficulties in designing effective p38a inhibitors as studies have so far focused on reduced conformations. Our results open up new avenues for the development of therapeutic compounds that modulate the activity of p38a more precisely."
Dr. Maria Macias, ICREA researcher and head of the Structural Characterization of Macromolecular Assemblies laboratory at IRB Barcelona
An oxidised form and a reduced form
The Protein Data Bank holds 357 structures of p38a protein, but they all correspond to its reduced form-;the only one known so far. The predominance of this form is possibly due to the prevalence of experimental conditions that include reducing agents in the structural studies carried out. In the oxidised form described in this study, a disulfide bridge is established, which forces a conformational change and blocks access to the binding site of activators and substrates. Thus, this is a new inactive form of p38a, which would be present in certain cellular conditions.
"The study of kinases in their oxidised forms is complex due to the influence of oxidative stress conditions and the transience of these forms in the cellular environment," explain Drs. Joan Pous and Pau Martin Malpartida and doctoral student Blazej Baginski, first authors of the study. "However, the key to addressing them effectively from a pharmacological perspective may lie in these forms," they conclude.
A promising approach
This new form illustrates a mechanism of action of p38a regulated by the cellular redox state, thereby explaining biochemical observations described to date but with no structural molecular basis.
In future work, the researchers will focus on exploring new interaction cavities that appear in the oxidised form as these may help to inactivate the protein withoutinterfering with the catalytic centre, thereby gaining specificity.
The work was developed in collaboration with Dr. Modesto Orozco's laboratory at IRB Barcelona and the University of Barcelona, and Nostrum Biodiscovery. The work received funding from the Spanish Ministry of Science and Innovation (MICINN), the European Research Council (ERC), the Catalan University and Research Grant Management Agency (AGAUR), and the BBVA Foundation.
Institute for Research in Biomedicine (IRB Barcelona)
Pous, J., et al. (2023). Structural basis of a redox-dependent conformational switch that regulates the stress kinase p38α. Nature Communications. doi.org/10.1038/s41467-023-43763-5.
Posted in: Molecular & Structural Biology | Cell Biology
Tags: Cancer, Cell, Chronic, Drugs, Enzyme, Inflammation, Laboratory, Oxidative Stress, Protein, Research, Stress