Study points to marketing of OxyContin as origin of the opioid epidemic
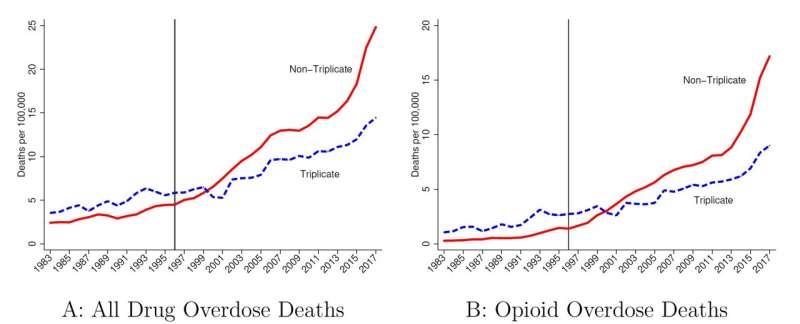
A state prescription drug policy first adopted in 1939, and last ended in 2004, appears to have influenced where Purdue Pharma chose to market its opioid drug OxyContin upon launch in 1996. The consequences of that decision reverberate to this day, according to a new study in The Quarterly Journal of Economics by LDI Senior Fellow Abby Alpert and colleagues, who estimate that states with the prescription policy had 11.3 fewer overdose deaths per 100,000 people in 2017.
The paper is a rigorous and tantalizing analysis of how public policy can influence and interact with commercial activities, leading to effects that could not be foreseen upon enactment of the policy. In the context of the opioid epidemic, these effects have had enduring impact on overdose deaths in the subsequent generation. These results are compelling evidence that the introduction and marketing of OxyContin explain a large share of the overdose deaths in the last 20 years.
The policy was an early prescription drug monitoring program that required physicians to use a state-issued triplicate form when prescribing Schedule II controlled substances (which includes many opioids). The physician had to keep one copy on record. The patient took two copies to the pharmacy; the pharmacy kept one and sent the third copy to the state drug monitoring agency. The state agency maintained a database from these forms to monitor and investigate prescribing irregularities and diversion. California was the first state to adopt a triplicate prescription program, followed by Idaho, Illinois, Indiana, Michigan, New York, and Texas between 1961 and 1988. Not surprisingly, all these programs eventually ended with the advent of electronic monitoring systems.
Enter Purdue Pharma, eager to market its new drug OxyContin in 1996. Triplicate programs had been effective in reducing prescribing of the drugs subject to the policy, due to physician concerns about tangible government oversight of their prescribing behavior and the hassle factor in handling and keeping records of the triplicate forms. Five states had a triplicate program in effect when Purdue Pharma crafted its marketing strategy (Indiana and Michigan had already ended their programs.)
“Triplicate states” are mentioned repeatedly in Purdue Pharma internal documents as having been important barriers to OxyContin prescribing, with lower expected returns from marketing spending. These documents suggest that less marketing was targeted to triplicate states, with one document recommending that “the product [OxyContin] should only be positioned to physicians in non-triplicate states.” Indeed, triplicate states had some of the lowest OxyContin adoption rates in the country.
Alpert and colleagues find that the marketing practices of OxyContin interacted with state policy and led to dramatically reduced overdose death rates in triplicate states. By comparing trends in triplicate vs. non-triplicate states, they provide empirical evidence that the origins of the opioid epidemic lie in the marketing of OxyContin. As shown in Figure 1, these differences became more pronounced as time went on, consistent with the ramp up in sales of OxyContin and time involved in transitioning from use to misuse and dependence.
Source: Read Full Article