Study explores adverse effects of SARS-CoV-2-induced antibody responses
Infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) triggers an immune response driven by the immunoglobulin G (IgG) family of antibodies in the infected individual. This response against the SARS-CoV-2 receptor-binding domain (RBD) is driven primarily by IgG1 and IgG3 sub-classes. While several studies have established the positive outcomes of this antibody-mediated viral neutralization, its potential adverse effects are yet unknown.
Study design
The authors of a research paper published in the journal Frontiers in Immunology used Sandwich ELISA tests to determine the distribution of IgG sub-classes and complement activation levels in plasma from convalescent individuals.
The study included a total of 180 people who recovered from coronavirus disease 2019 (COVID-19), as confirmed by their negative reverse transcription-polymerase chain reaction (RT-PCR) test. Plasma samples from these individuals were ethylenediaminetetraacetic acid (EDTA)-treated and kept frozen at −80°C until they were used.
The RT-PCR positive participants comprised males and females in the age group 18–86, with the course of disease ranging from mild (few symptoms) to severe (needed hospitalization). Healthy blood donors were used as negative controls to collect a total of 60 EDTA plasma samples (from before 2020) having no SARS-CoV-2 antibodies.
Assessing levels of IgG sub-classes and complement molecules
During natural infection, IgG1 and IgG3 sub-classes effectively activate the classical complement pathway to eliminate the pathogen (here SARS-CoV-2) employing methods such as microbial lysis by the terminal complement complex (TCC).
The complement system, an integral part of innate immunity, is initiated by three distinct pathways – the classical, the lectin, and the alternative pathway – differing in the modes of initiation but all generating complement factor (C3) and other central and terminal effector molecules.
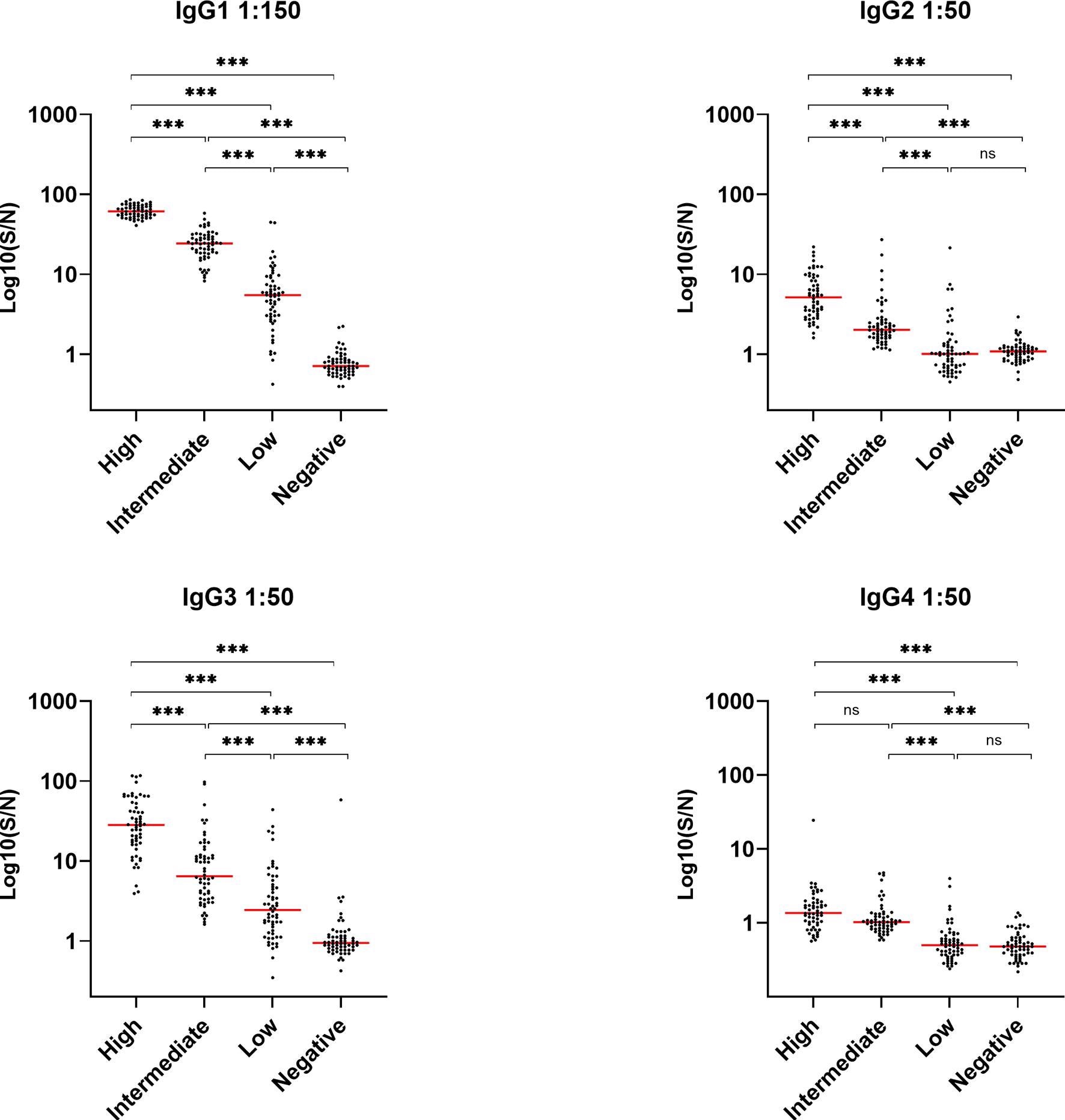
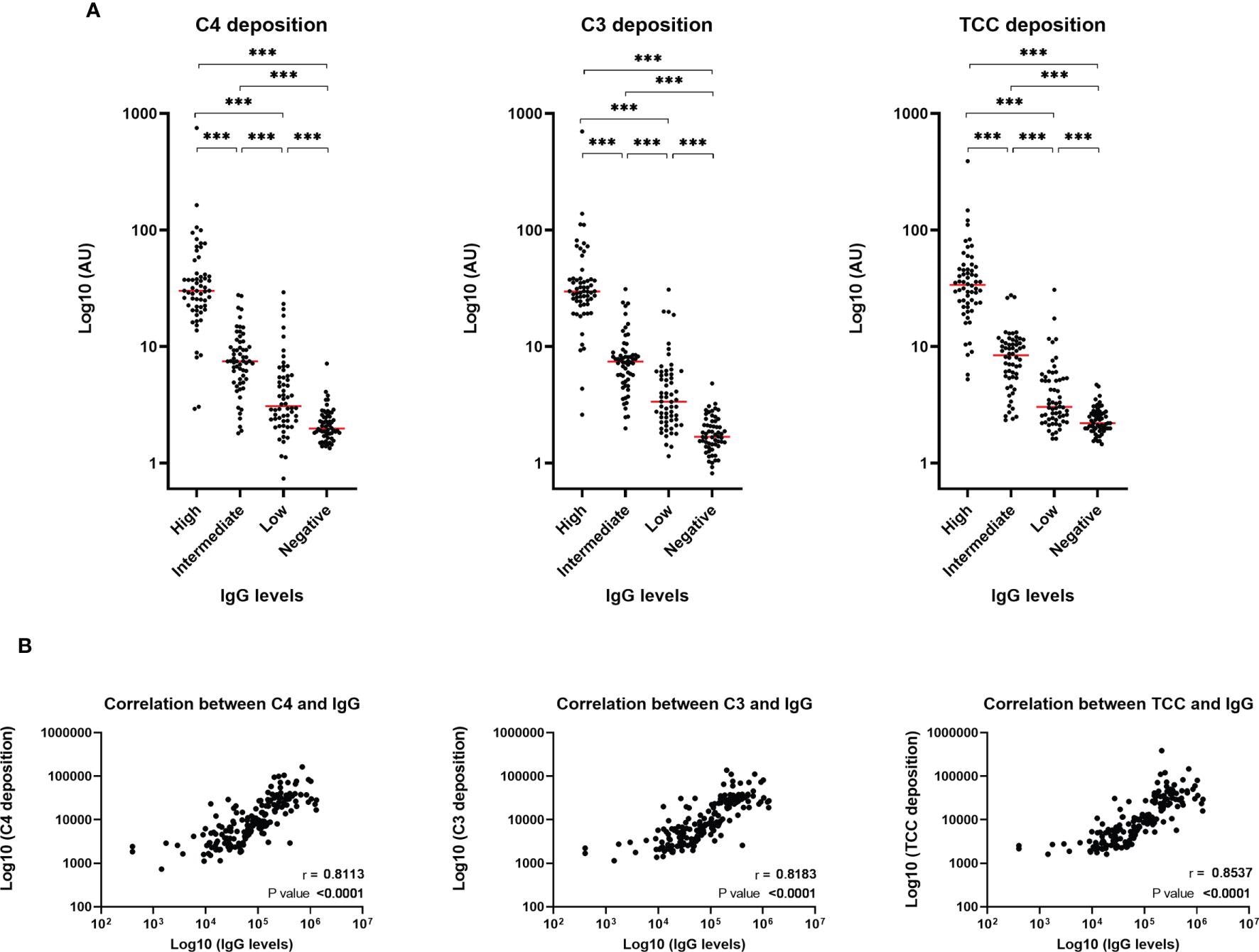
Deposition of such effector molecules (C4b, C3bc, and TCC), as a consequence of SARS-CoV-2-specific antibodies, are significantly correlated with both IgG levels and disease severity, indicating that patients with high IgG levels might have more prominent complement activation during viral infection.
Complement activation – a distinctive pathogenic feature of COVID-19
While the activation of the complement system may effectively contribute to the control of infection, it may also contribute to several pathologies like acute respiratory distress syndrome (ARDS) in severely ill COVID-19 patients due to its pro-inflammatory effects.
The study results suggest that antibody-mediated complement activation and subsequent cellular priming constitute a significant risk of increasing COVID-19 severity. In addition, the results indicate a preferential generation of SARS-CoV-2-specific IgG3 (and not IgG1) during COVID-19 infection, while IgG2 and IgG4 are barely detected. This excess IgG3 could be responsible for excess inflammation rather than tissue repair, worsening the COVID-19 symptoms in patients.
Inferences drawn from the depletion of antibodies against RBD
The results demonstrated that once the RBD antibodies depleted in convalescent plasma samples, the deposition of C4, C3 and TCC complement molecules stopped. However, while this proved that the activation of the complement system through deposition of C4, C3, and TCC is mediated by RBD antibody, the results could not decipher the exact residues of the RBD involved in triggering the complement activation.
Furthermore, the levels of C4, C3, and TCC are positively correlated with both IgG levels and disease severity. Both C4 and C3 levels were significantly lower in patients with high disease severity than patients with low severity, indicating that C4 and C3 are readily absorbed in severely ill patients.
Cytokine production in monocytes
An aggressive cellular mediated inflammatory response observed in SARS-CoV-2-infected individuals is a consequence of Fc – gamma receptor (FcγR) engagement by SARS-CoV-2 specific antibodies. Simultaneously, it releases heaps of pro-inflammatory cytokines, referred to as the "cytokine storm." While studies have investigated the role of antibodies and FcγRs during viral infections concerning virus neutralization and antibody-dependent enhancement of infection, data on FcγR-mediated cytokine responses in the context of viral infections is limited.
To fill this knowledge gap, researchers primed THP-1 monocytes and freshly isolated human monocytes with SARS-CoV-2 RBD immune complexes, the antigen being RBD. Upon evaluating the cytokine secretion of IL-6, IL-1β, and TNF-α by THP-1 cells, observed TNF-α levels were significantly high, but IL-6 and IL-1β levels were not. The monocytes and monocyte-derived macrophages are phagocytic cells contributing to inflammation, immunity, and anti-viral responses.
Future directions
The study's findings provide an essential insight into the overall immunological response during a COVID-19 infection and emphasize the need for further studies to shed light on all the possible harmful effects of these antibodies and their interaction with the complement system.
- Ida Jarlhelt, Sif Kaas Nielsen, Camilla Xenia Holtermann Jahn, Cecilie Bo Hansen, Laura Pérez-Alós, Anne Rosbjerg, Rafael Bayarri-Olmos, Mikkel-Ole Skjoedt and Peter Garred. SARS-CoV-2 Antibodies Mediate Complement and Cellular Driven Inflammation. Front. Immunol. (2021). DOI:https://doi.org/10.3389/fimmu.2021.767981, https://www.frontiersin.org/articles/10.3389/fimmu.2021.767981/full
Posted in: Medical Research News | Disease/Infection News
Tags: Acute Respiratory Distress Syndrome, Antibodies, Antibody, Antigen, Blood, Convalescent Plasma, Coronavirus, Coronavirus Disease COVID-19, Cytokine, Cytokines, Immune Response, immunity, Immunoglobulin, Immunology, Inflammation, Monocyte, Pathogen, Polymerase, Polymerase Chain Reaction, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Transcription, Virus

Written by
Neha Mathur
Neha Mathur has a Master’s degree in Biotechnology and extensive experience in digital marketing. She is passionate about reading and music. When she is not working, Neha likes to cook and travel.
Source: Read Full Article