Nanoparticles elicit neutralizing antibodies to diverse SARS-CoV-2 variants
Spike-protein-based nanoparticle vaccine candidates were retained longer in follicular dendritic cells and showed higher germinal center reactions in lymph node follicles compared to soluble spike protein vaccine.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected hundreds of millions worldwide since its emergence more than a year ago. The virus has also been mutating throughout the pandemic, with several new variants, likely more infectious, being reported over the last few months.
To combat SARS-CoV-2, several vaccines have now been approved, and more than 100 are at different stages of clinical trial. However, the approved vaccines have shown a reduced efficacy against the new variants, which have mutations on the virus spike protein, N-terminal domain, and other regions.
The B.1.351 variant appears to be more resistant to convalescent sera and sera from vaccinated people compared to the B.1.1.7 variant. This suggests the need to develop vaccines with a broad neutralizing antibody response against different variants.
Broad antibody responses need long-lived germinal center (GC) reactions to activate precursor B cells and form long-term immune memory. Long-lived GC reactions can be maintained by antigen retention in lymph node follicles, which can be used to develop broad antibody response vaccines.
Previously, researchers designed a spike protein on three self-assembling protein nanoparticles as SARS-CoV-2 vaccine candidates. In a new study published on the bioRxiv* preprint server, researchers tested these against SARS-CoV-2 variants.
Nanoparticle vaccines retained in lymph nodes
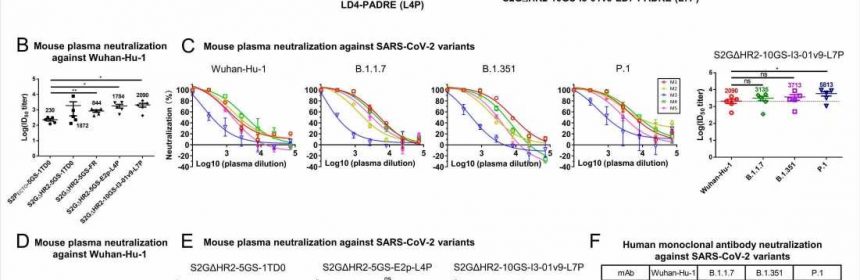
The authors found that all three nanoparticle-based vaccines showed higher levels of neutralizing antibodies compared to a control vaccine, by up to 8-fold, in mice sera. One of the three also showed higher antibodies against the new variants: 0.5-fold for B.1.1.7, 0.8-fold for B.1.351, and 1.8-fold for P.1, compared to the Wuhan-1 strain. All three variant pseudoparticles were neutralized by the mouse sera.
The team found that the route of injection also affected antibody levels. When the vaccines were injected intradermally on the mouse footpads, the vaccines that had about 20 spikes gave higher neutralizing antibody levels than the soluble version. This suggests a higher nanoparticle display is necessary for eliciting a broad antibody response.
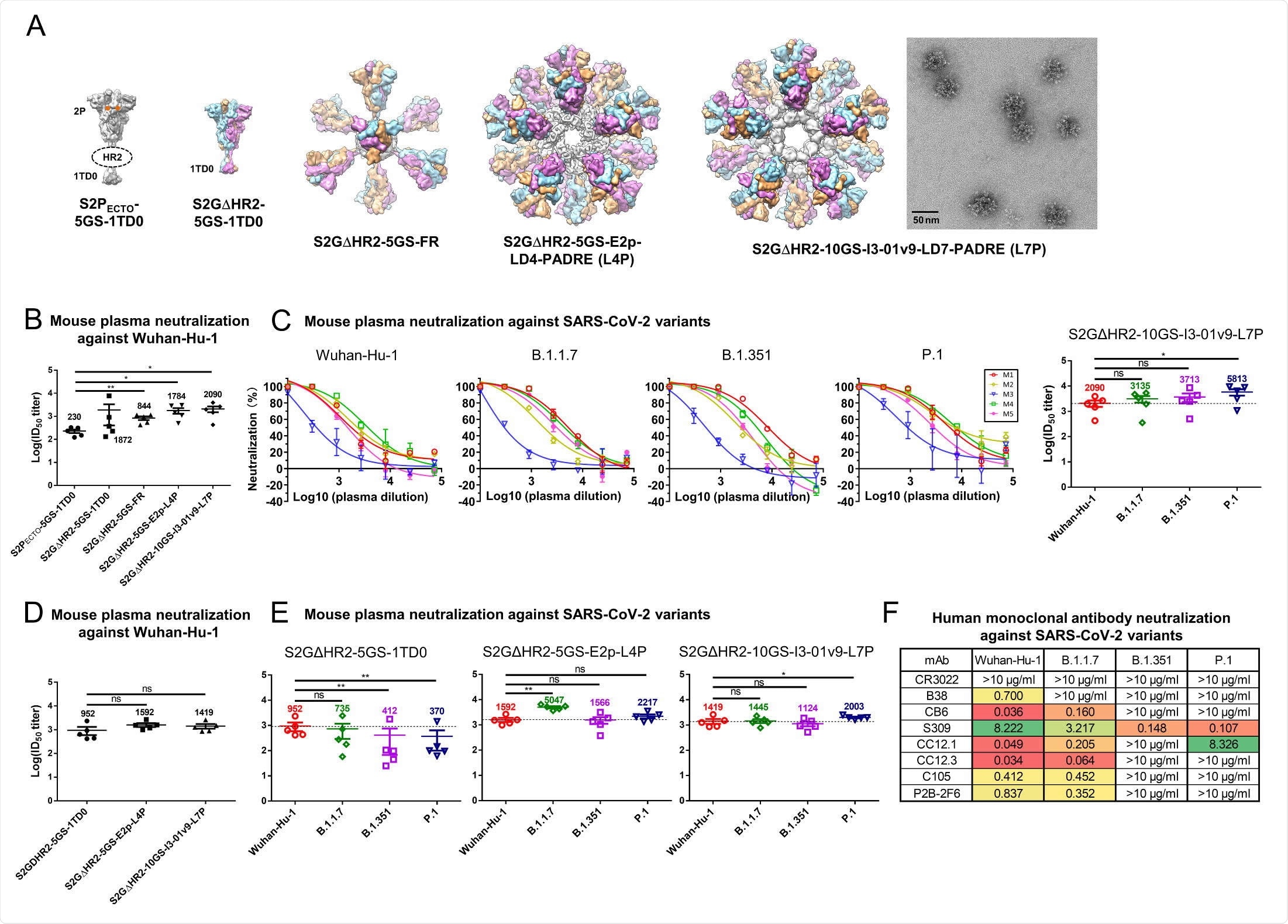
To understand why the nanoparticle spikes show better performance than the soluble spikes, the researchers tested the soluble and nanoparticle vaccines in mice by injecting them into footpads. Upon analyzing the lymph nodes, they found that the nanoparticles-based spikes accumulated in the lymph node follicles, with their distribution in them differing slightly based on the number of doses.
The control vaccine entered lymph nodes within two hours and were gone by 48 hours. However, the two large nanoparticle vaccines entered the follicles about 12 hours after injection and were detected up to two weeks, indicating greater retention than the control. This is similar to previous studies that reported smaller nanoparticles cleared quickly while large nanoparticles were retained for a longer time.
Lymph nodes retain larger particles
Further tests revealed that the follicular dendritic cells (FDCs) play a crucial role in the retention of the vaccine in the lymph nodes. Using transmission electron microscopy, the team looked at the interface between FDCs and B cells. They found intact spike nanoparticles on and in macrophages inside lymph nodes, suggesting FDCs can help retain the spike nanoparticles.
The team also found that the spike nanoparticles vaccines induced strong, long-lived GCs, while the soluble spike did not sustain GCs eight weeks after vaccination, either with a single dose or with a booster dose. The nanoparticles showed up to a five-fold increase in GC B cells and T follicular helper cells compared to the soluble spike vaccine.
The results showed that the nanoparticle vaccine candidates showed six-fold longer retention and four-fold more accumulation in lymph nodes than the soluble spike protein candidate. It is likely this is because of the properties of lymph nodes that help retention of larger particles. The nanoparticle candidates are uniquely suited for producing long-lived GCs in lymph nodes.
Although protein vaccines have long been used with reasonable safety and efficacy, they have yet to be used for SARS-CoV-2. Protein vaccines alone or as boosters for nucleic acid vaccines. The team found that the spike nanoparticle vaccines adjuvanted with AddaVax and aluminum phosphate induced stronger GC reactions than vaccines without adjuvants. Further testing of adjuvants and a broader understanding of how nanoparticle vaccines and other platforms behave in the body will help develop vaccines more quickly.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Zhang, Y.-N. et al. (2021) Mechanism of a COVID-19 nanoparticle vaccine candidate that elicits a broadly neutralizing antibody response to SARS-CoV-2 variants, bioRxiv, https://doi.org/10.1101/2021.03.26.437274, https://www.biorxiv.org/content/10.1101/2021.03.26.437274v1
Posted in: Medical Research News | Disease/Infection News
Tags: Antibodies, Antibody, Antigen, Clinical Trial, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Electron, Electron Microscopy, Lymph Node, Lymph Nodes, Microscopy, Nanoparticle, Nanoparticles, Nucleic Acid, Pandemic, Protein, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, Virus

Written by
Lakshmi Supriya
Lakshmi Supriya got her BSc in Industrial Chemistry from IIT Kharagpur (India) and a Ph.D. in Polymer Science and Engineering from Virginia Tech (USA).
Source: Read Full Article