How safe are breast implants? Women warn of risks, share their symptoms
Judith Coates felt like she had “won the lottery” when she was told that an implant could replace the breast she was losing to cancer.
“It was like, ‘This is the new improved me,’” she said about her combined mastectomy and implant surgery, which removed her right breast and added in a silicone implant. “I was really happy about it.”
Six months later though, she said she started feeling a “clenching” tight feeling in her chest. She also started dropping things. “I just thought I was being a klutz and then I realized I didn’t have any feeling in my hand.”
She also had bad tendinitis, then later, blurry vision and trouble swallowing.
“And I started looking up some of these symptoms and that’s what led me to a Facebook group that had a lot of women in it that were having the same symptoms and they had breast implants. So I put two and two together.”
Related
 ‘They should be better warned’: Canadian doctor on breast implant risks
‘They should be better warned’: Canadian doctor on breast implant risks
 Health Canada moving to ban textured breast implants over cancer risk
Health Canada moving to ban textured breast implants over cancer risk
 Breast implant safety under review by U.S. authorities
Breast implant safety under review by U.S. authorities
She had the implant removed in October 2018, a little less than three years after she had it put in.
“Immediately when I woke up, I could take a deep breath. I felt like I had my lung capacity back again,” she said. “I didn’t get winded and then a few of the other symptoms went away immediately as well.”
Coates still has a few issues, she said, but she estimates that around 90 per cent of her problems disappeared along with the implant.
Coates and many other women who had breast implants decided to take action. She’s been active on Facebook, and recently, put her name on a petition that was presented with 3,448 signatures in the House of Commons in early April.
The petition asks Health Canada to investigate the links between implants and a rare form of cancer, as well as autoimmune diseases.
Some of Pierre Blais’ collection of defective breast implants, many of which are no longer in production.
Breast implant-associated large cell lymphoma
Health Canada announced in mid-April that it was notifying a breast implant manufacturer, Allergan, that it intended to cancel its licence to sell its Biocell brand of textured silicone implants – effectively removing them from the Canadian market.
This was due, said the department, to a link between these implants and a rare form of cancer called breast implant-associated large cell lymphoma.
This cancer isn’t of the breast tissue but develops in a fluid near the implant and in some cases can spread throughout the body.
A textured breast implant.
As opposed to a smooth-surfaced implant, a textured implant “carries with it a measurable but very low risk of lymphoma – a type of tumour – not in the breast implant itself, but it’s based on a reaction of the surrounding tissue to the implant,” said Dr. Nick Carr, a clinical professor at the University of British Columbia and ex-chair of the school’s division of plastic surgery.
The FDA warned about the risks and held hearings in March, though it decided in early May that it wouldn’t be banning the implants.
Breast implant illness
Symptoms like fatigue, joint pain and cognitive impairment reported by women like Coates, are often grouped under the heading “breast implant illness,” and are harder to link to the implants as they’re not an officially recognized syndrome.
An early model of breast implant, made of foam.
“I think the majority of plastic surgeons are aware of the concerns about breast implant illness,” Carr said. “I would say the position of most plastic surgeons certainly in Canada is that the evidence for the implants being the cause of some of these symptoms is not terribly good and that a lot of the complaints that these women have are reasonably subjective and hard to sort of pin down as being attributable to anything specific.”
Dr. Jan Willem Cohen Tervaert would disagree. “There is a long debate about breast implant illness. Is it a true disease or is it just that women made it up?”
Tervaert, who is a professor of medicine at the University of Alberta, believes that the reports have been consistent enough – women reporting the same kinds of symptoms now as women with breast implants did decades ago – to show that there is some risk.
“The second argument is that if you remove the breast implants, the symptoms disappear,” he said.
That was Rose Finlay’s experience. The Bowmanville, Ont., woman said she suffered a seizure shortly after getting implants, with no explanation given. She also spent time in hospital.
“I was on my deathbed,” she said. “I was going to die and there was no explanation. My kidneys were shutting down. My pee was black. I was not eating. I couldn’t keep anything down. I had chronic diarrhea. I was losing weight alarmingly.”
A defective breast implant, where the casing is flaking apart.
But when she decided to get the implants taken out, things got better. “About 90 per cent of my symptoms went away as soon as I got them removed.”
Breast implants are not a lifetime product – women will need to have them replaced every 10-20 years or so.
And there are other risks too. Aside from the general risks of any surgery, as the tissue around the implant heals, it creates a “capsule” of scar tissue surrounding the implant. If that grows improperly, it can distort the implant or make the breast feel harder to the touch – a condition known as “capsular contracture.”
Pierre Blais has seen a lot of defective implants. The Ottawa resident and former federal health employee runs a business called Innoval Failure Analysis, that analyzes medical devices including breast implants and reports on defects found within them.
Essentially, women or their families send him their used implant, in hopes that he can discover what went wrong. His reports are then used in court cases or for the patients’ own information. He estimates he’s analyzed about 20,000 implants from around the world.
A breast implant, where the woman’s body developed a hard shell around it, according to Pierre Blais.
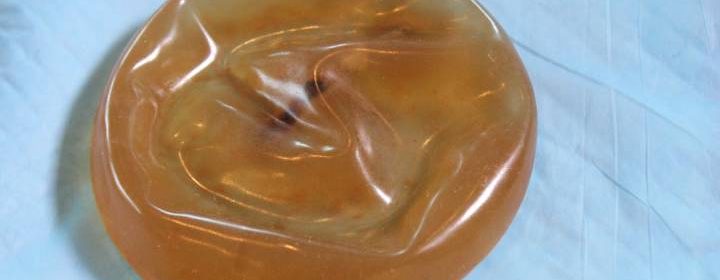
Some common issues he describes are valves in saline implants not closing properly, allowing bodily fluids and bacteria to contaminate the liquid inside – in some cases turning the clear fluid a murky brown.
He’s also seen the outer casing of some implants flake off, he said, leaving bits of the shell inside the body. Sometimes calcium deposits form around the implants, he said, again leaving bits behind.
“The first question [clients] ask is, ‘Is my implant contaminated? What did it contain? How come I am sick? I feel as if I’ve been poisoned.’ That’s the question.”
He doesn’t think implants are safe and he warns that patients who have them and develop symptoms may not be listened to by their friends or even physicians.
Finlay admits that she’s had trouble at times getting sympathy for what was a bit of plastic surgery that she chose to do, rather than something which was medically necessary.
But, she’d like patients to be made more aware of the risks, and the government to be more active in regulating the products.
“We weren’t warned,” she said. “You’re told by a medical professional this is safe.”
“They don’t tell you, ‘Oh you know this might poison you and it might ruin your life.’ And I mean, it’s like that with any surgical procedure but going into anything else, you feel more protected whereas here it’s like, ‘Oh well, you guys chose this. So sorry. It was a faulty product.’ And so that’s I think what feels really wrong to me.”
Sign up for our Health IQ newsletter
© 2019 Global News, a division of Corus Entertainment Inc.
Source: Read Full Article
-PKG-online_848x480_1508531779871.jpg)



