Cross-reactive coronavirus antibodies might not be cross-neutralizing
In a recent study posted to Research Square* preprint server, researchers reported that cross-reactive antibodies against coronaviruses (CoVs) might not cross-neutralize all CoVs.
Several studies have reported that, in binding and pseudovirus neutralization assays, exposure to CoVs or vaccination against them induces cross-reactive antibodies, including against the latest severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, plasma from the pre-coronavirus disease 2019 (COVID-19) pandemic times could not neutralize live SARS-CoV-2.
Occasionally, the results of binding assays are referred to as neutralizing, but the relevance of binding assays is different from the neutralization of viruses by antibodies. For example, recent studies have reported that antibodies against SARS-CoV-2 could bind to seasonal CoVs, and it remains unclear whether these cross-reactive antibodies could neutralize the seasonal CoVs.

About the study
In the present study, researchers evaluated whether SARS-CoV-2 infection or vaccination-induced immune responses could cross-neutralize seasonal CoVs.
Antibody concentrates were prepared from the plasma of SARS-CoV-2-naïve donors (pre-pandemic), plasma from COVID-19-recovered donors (post-COVID), or vaccinated donors (pandemic). The collected plasma specimens were used to prepare immunoglobulin (IG) lots through a licensed process, which were then tested to neutralize SARS-CoV-2 and human CoVs (HCoVs) such as OC43 and NL63. The pre-pandemic and pandemic IG were prepared from the plasma of donors in the United States (US) from April to June 2020 and July to September 2021, respectively. Post-COVID IG was obtained from donors from Austria or the US.
A previously established protocol determined neutralizing antibody (nAb) titers against SARS-CoV-2. Similarly, nAb titers against both HCoVs were evaluated. In brief, the serially diluted (IG) specimens were incubated with either HCoV at 103 median tissue culture infectious dose (TCID50) per milliliter (ml). The cytopathic effect was studied after 9 – 11 days of incubation of HCoV-NL63 on LLC-MK2 cells and 6 – 8 days of HCoV-OC43 on MRC-5 cells. The resultant 50% virus neutralization (µNT50) was estimated using the Spearman-Karber method and subsequently normalized to an internal control. The nAb potency was compared between pre-pandemic, pandemic, and post-COVID IG lots.
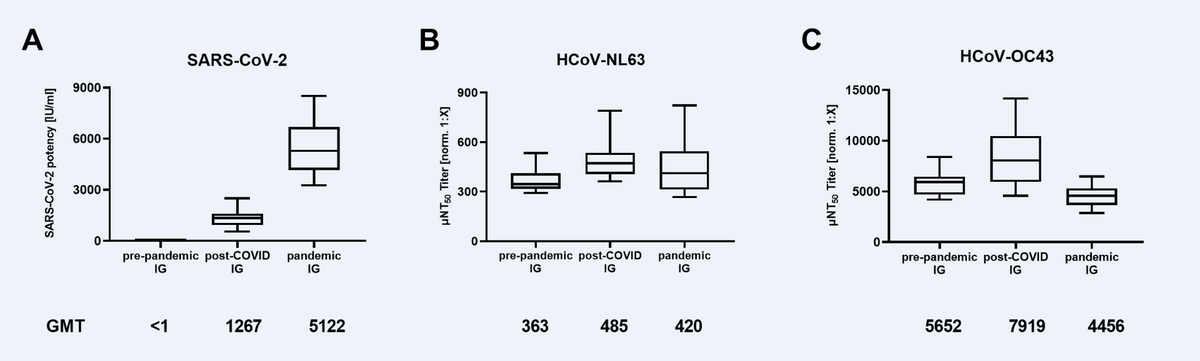
Neutralizing antibody titers in immunoglobulin (IG) lots manufactured from pre-pandemic plasma (N=16), plasma of post-COVID-19 individuals (post-COVID, N=21), and plasma of mostly COVID-19 vaccinated donors (pandemic, N=18) against (A) SARS-CoV-2, (B) Human Coronavirus NL63 (HCoV-NL63) and (C) HCoV-OC43. Box plots show medians with 25th to 75th percentile ± min/max as whiskers. Geometric mean titers (GMT) are indicated below the x-axis.
Findings
About 363 IG lots were characterized for nAbs against SARS-CoV-2 and HCoVs. None of the IG lots prepared from pre-pandemic donors neutralized SARS-CoV-2. The post-COVID IG exhibited a mean neutralizing activity of 1267 international units (IU) per ml, lower than the mean nAb titers (5122 IU/ml) of pandemic IG lots. In contrast, the pre-pandemic IG neutralized HCoV-OC43 (363 µNT50 geometric mean titer [GMT]) and NL63 (5652 µNT50 GMT). The neutralization of both HCoVs was comparable across the three types of IG preparations.
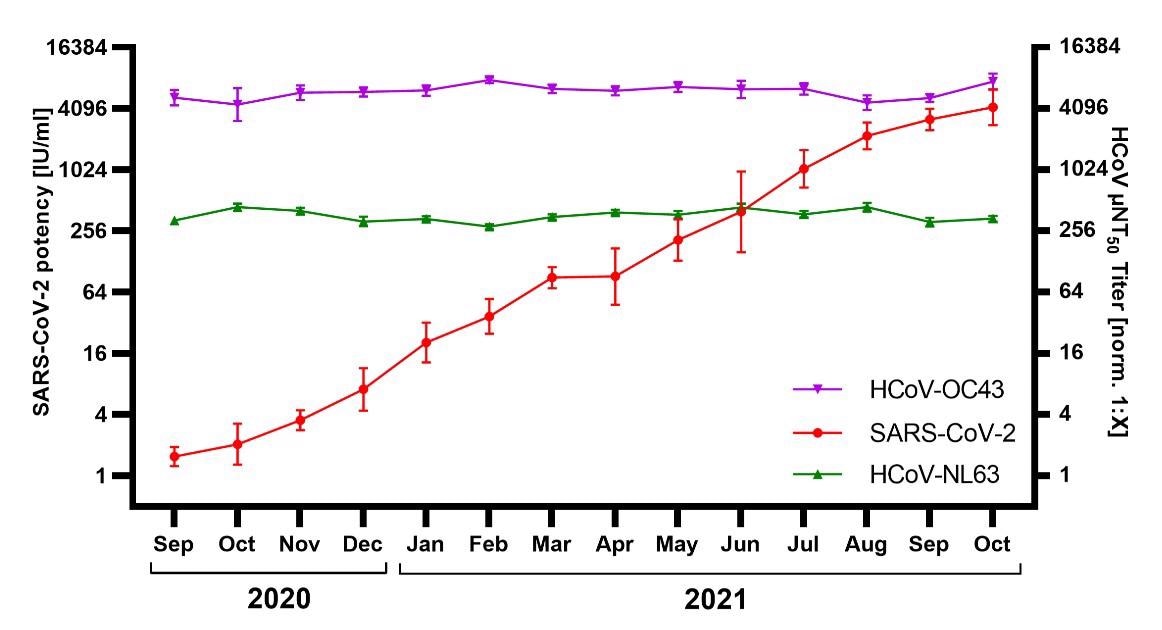
From September 2020 to October 2021, about 326 IG lots were prepared and screened for nAbs against SARS-CoV-2 and HCoVs. The IG preparations from September 2020 had a mean nAb titer of 2 IU/ml against SARS-CoV-2, which increased to 4210 IU/ml for IG lots prepared in October 2021. Nevertheless, the nAb titers against HCoVs remained relatively stable during this period.
Neutralization of SARS-CoV-2, HCoV-NL63, and HCoV-OC43 by immunoglobulin (IG) released September 2020 – September 2021 (N=326; 13–31 lots / month). SARS-CoV-2 neutralization was normalized to WHO International Standard 20/136 and reported as international units / milliliter (IU/ml). HCoV titers were normalized to an internal standard and reported as µNT50 titer [norm. 1:X]. Shown are geometric mean titer ± 95% confidence intervals.
Conclusions
Consistent with several other reports, the authors did not observe SARS-CoV-2 neutralization by IG preparations from plasma of pre-pandemic donors. Although post-COVID IG lots potently neutralized SARS-CoV-2, IG preparations from vaccinated donors were nearly 4-fold more potent. The pre-pandemic IG neutralized HCoVs with high potency.
In contrast, post-COVID and pandemic IG lots significantly and potently neutralized SARS-CoV-2. The neutralizing activity of post-COVID and pandemic IG against HCoVs was comparable to that of pre-pandemic IG lots.
The current study's findings suggested that antibody binding or pseudovirus neutralization assay results might not necessarily reflect the neutralization of live viruses.
Notably, plasma from a SARS-CoV-2-infected or vaccinated donor contains both IgG and IgM, while commercial IG preparations only concentrate IgG, which might explain the observed results. The authors posit that IgM might be cross-neutralizing while IgG, despite being cross-reactive between seasonal CoVs and SARS-CoV-2, might not be cross-neutralizing.
*Important notice
Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Function matters: Coronavirus cross-binding antibodies do not cross-neutralize, Maria R. Farcet, Julia Schwaiger, Michael Karbiener et al. 20 April 2022, PREPRINT (Version 1) available at Research Square, DOI: https://doi.org/10.21203/rs.3.rs-1576167/v1, https://www.researchsquare.com/article/rs-1576167/v1
Posted in: Medical Research News | Disease/Infection News
Tags: Antibodies, Antibody, Assay, Coronavirus, Coronavirus Disease COVID-19, Immunoglobulin, Pandemic, Pseudovirus, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Tissue Culture, Virus

Written by
Tarunpreet Singh Virk
Dr. Tarunpreet Singh Virk, was born in Tarn Taran city of Punjab, India. He obtained his B.Sc. (HS) and M.Sc. (HS) degrees from GNDU, India and subsequently completed his PhD in Organic Chemistry from the same University in 2015. Dr. Tarunpreet Singh Virk held a postdoctoral research position at the University of Ottawa, Canada from Nov 2015 to Oct 2016 and subsequently completed another postdoctoral stint at IISER Pune from Aug 2017 to Jan 2019. He had also served on the position of Assistant Professor of Chemistry in colleges.
Source: Read Full Article