can prevacid cause hives
Buy generic alesse online pharmacy without prescription
Skip to:
- What is Klotho?
- Physiological Roles of Klotho
- Summary
What is Klotho?
The klotho (kl) gene was identified as a regulator of normal ageing, where mutated forms led to accelerated ageing phenotypes. Therefore, klotho can be considered as an ‘ageing-suppressor’ gene. In ancient mythology, Klotho, or Clotho, was a daughter of the ancient Greek God Zeus and member of the three fates who was responsible for spinning the thread of life.
The klotho gene can be found on chromosome 13 and it encodes a transmembrane protein that controls the sensitivity of cells to insulin and involved in ageing. The gene contains 5 exons and encodes a 1012 amino acid length single-pass transmembrane glycoprotein in the plasma membrane and the Golgi apparatus. The intracellular domain does not contain any functional domains whereas the extracellular domain contains 2 repeats; KL1 and KL2. Klotho can be found expressed in various cell types and tissues, but predominantly high expressions within the kidney and choroid plexus of the brain. Moderate expression can also be found in the parathyroid gland, parts of the aorta and sex organs.
Physiological Roles of Klotho
There are different forms of Klotho: membrane-bound, intracellular and secreted.
Membrane-bound Klothos can form complexes with FGFRs (fibroblast growth factor receptors) to increase their affinity to FGFs, especially FGF23, FGFG15 & FGF19 (roles discussed later). Intracellular Klotho can bind to Na+-K+-ATPase to stimulate its surface activity in intracellular organelles. Furthermore, intracellular Klotho is able to supress RIG-1 (retinoic acid inducible gene 1) acting as an anti-inflammatory and anti-ageing factor. Secreted Klotho acts as a humoral factor principally acting as an anti-ageing and protective agent by targeting growth factors and cytokine signalling. Specific roles are discussed in more detail below.
Inhibition of the Insulin/IGF-1 Pathway: Klotho knockout mice have shown to have a decreased insulin production with increased insulin sensitivity. As such, null mice have less energy storage and expenditure than controls. Klotho can inhibit IGFRs and IRS (insulin receptor substrate) through indirect mechanisms.
Activation of IRS normally leads to the activation of PI3K/Akt pathway leading to the phosphorylation of FOXO1, FOXO3a and FOXO4, which subsequently remain in the cytoplasm rather than the nucleus leading to a loss of its transcriptional activity. FOXO’s are normally involved in the regulation of gluconeogenesis (glucose production) and glycogenolysis via insulin signalling in hepatocytes, as well as important for committing preadipocyte to undergo adipogenesis.
Suppression of WNT glycoprotein signalling: Klotho is able to bind to many Wnt ligands to supress Wnt signal transduction. This is evidenced by the fact that Klotho null mice display enhanced Wnt signalling. Normally Wnt3 signalling, for example, is able to arrest the cell cycle at G2/M phase therefore prolonging it. Klotho treated cells bypass this cell cycle phase.
Within the kidney, under hypoxic conditions, Klotho is inhibited whereas β-catenin is upregulated. Therefore, Klotho may actually be a natural antagonist of endogenous Wnt/β-catenin signalling, and a loss of Klotho may lead to kidney disease. Expressing Klotho is able to decrease the activation of kidney β-catenin to improve kidney function.
Calcium & Phosphate Homeostasis: Soluble Klotho is able to increase the abundance and expression of a calcium channel, TRPV5 on the plasma membrane. This is achieved by blocking the endocytosis of TRPV5 by stabilising it via a sialidase function to remove 2,6-sialylated LacNAc from the TRPV5 N-glycosylation branch to expose galectin-1 binding site. Klotho is also able to increase the plasma membrane stabilisation and retention of TRPV2, which can lead to enhanced insulin secretion in pancreatic β-cells.
Klotho is also able to bind to TRPC1 (transient-receptor potential canonical Ca2+ channel 1) via its KL2 domain to regulate TRPC1 mediated Ca2+ flux entry to maintain endothelial integrity. Klotho is also able to form a complex with the Na+,K+-ATPase to increase its expression at the plasma membrane under an increase to physiological extracellular Ca2+.
Phosphate (Pi) levels are regulated by FGF23 (as discussed earlier), amongst other enzymes. Pi absorption leads to an increased secretion of FGF23 (gene associated with rickets). FGF23 is needed to regulate the renal excretion of Pi. Klotho can bind to FGFRs to increase the binding affinity of FGF23, which typically has a low affinity to FGFRs. Upon binding, Klotho is able to activate the MAPK pathway to inhibit Pi reabsorption in the kidney. As such, Klotho knockout mic display a large increase in serum levels of Pi and Ca2+, in addition to premature ageing phenotypes. This is because hyperphosphatemia can promote ageing in mammals.
Oxidative Stress Suppression: Klotho can upregulate FOXO3a phosphorylation. FOXO31 itself upregulates MnSOD (manganese superoxide dismutase) expression; a mitochondrial antioxidant enzyme, which in turn lowers mitochondrial ROS (reactive oxygen species) production. Mice that overexpress Klotho have higher levels of MnSOD and lower oxidative stress as evidenced by lower levels of DNA damage. Overexpressing Klotho can reduce the levels of peroxide induced apoptosis, mitochondrial DNA fragmentation and superoxide anion generation.
Summary
Klotho, in its various forms, can regulate mineral metabolism via FGF23, suppress Wnt signalling, modify TRPV5 mediated calcium signalling, as well as inhibit the activation of the IGF/insulin signalling pathway.
Furthermore, Klotho is also able to promote cellular resistance to oxidative stress. As such, Klotho can effectively slow down the process of ageing and extend lifespan, an ageing-suppressor gene. The mechanisms behind the effects of Klotho, or indeed identification of its different forms and their interactions, is still poorly understood thus the lack of progress in Klotho based therapies.
Sources
- Kim et al, 2015. Biological Role of Anti-aging Protein Klotho. J Lifestyle Med. 5(1): 1–6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4608225/
- Xu & Sun, 2015. Molecular Basis of Klotho: From Gene to Function in Aging. Endocr Rev. 36(2): 174–193. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4399270/
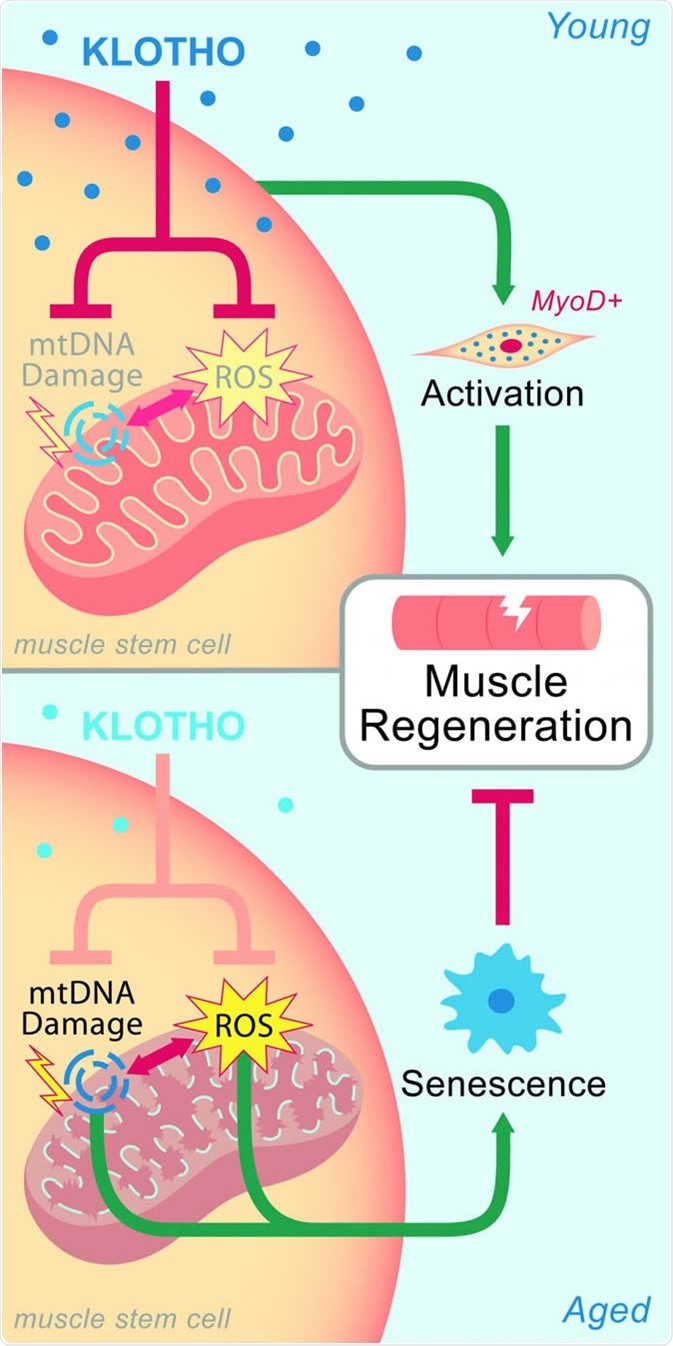
- Sahu, A., Mamiya, H., Shinde, S.N. et al. Age-related declines in α-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat Commun 9, 4859 (2018). https://doi.org/10.1038/s41467-018-07253-3
Further Reading
- All Chromosome 13 Content
Last Updated: Feb 6, 2020

Written by
Osman Shabir
Osman is a Neuroscience PhD Research Student at the University of Sheffield studying the impact of cardiovascular disease and Alzheimer's disease on neurovascular coupling using pre-clinical models and neuroimaging techniques.
Source: Read Full Article