A molecular switch for stomach disease
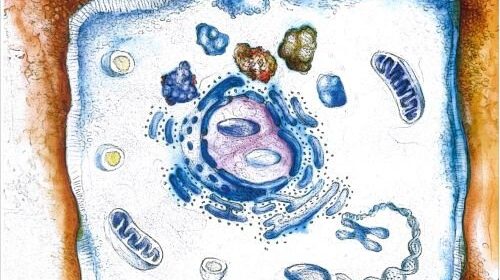
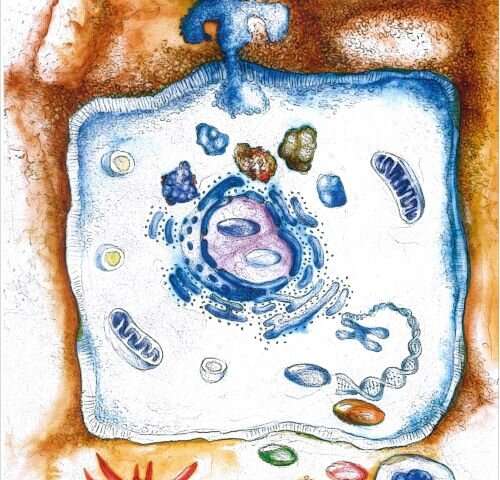
Infectious diseases triggered by bacteria and other microbes are the most frequent cause of human mortality around the globe. Roughly half of the world’s population carries the stomach-infecting bacteria Helicobacter pylori (H. Pylori), which is known to be the most significant risk factor for ulcers, MALT lymphoma and adenocarcinoma in the stomach. The rapid spread of pathogens resistant to antibiotics is making it increasingly difficult to treat infections like these using antimicrobial therapies. A research team from Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) has now revealed a new mechanism that controls the causes of infection with H. pylori. These findings could eventually lead to new therapies. The study was published in the journal Nature Communications.
A team of national and international scientists led by Prof. Dr. Steffen Backert from the Chair of Microbiology at FAU has investigated how the bacteria manipulate the host’s immune system in order to ensure their long-term survival in the stomach. A chronic inflammation is the most common cause for stomach illnesses such as these. The researchers have identified a molecular switch that uses a previously unknown mechanism to regulate the inflammation reaction in the stomach.
The interaction between H. pylori and stomach cells activates a syringe-like pilus structure referred to as a type IV secretion system. A protein, CagL, is presented at the surface of this structure and allows the bacterial toxin CagA protein to be delivered into the stomach cells. The injected CagA then re-programs the host cell so that stomach cancer can develop. It now appears that CagL also has another important function, however. The protein is recognized by the immune system via the receptor TLR5. Experiments in mouse models have demonstrated that TLR5 controls the infection efficiently. CagL imitates a TLR5 recognition motif in the flagellin protein of other pathogens, thereby controlling the human immune response.
Interestingly, this signaling pathway can be both switched on and switched off by the type IV secretion system, which is not thought to be the case with other bacteria. Presumably, H. pylori has exploited this signaling pathway over thousands of years of evolution to eliminate “bothersome” bacterial competitors in the stomach. At the same time, CagL influences the congenital and adaptive immune system as well as the inflammation reaction in such a way that H. pylori itself is not recognized and therefore cannot be eliminated—a mechanism crucial for long-term H. pylori infections in the stomach and for triggering stomach disease. Researchers also observed that TLR5 is no longer produced in healthy stomach cells, or in stomach cells after an infection has been resolved. This indicates that the expression of this protein is a new indicator for stomach disease in humans triggered by H. pylori.
Source: Read Full Article