Using the immune system, hydrogels, and bacteria to treat and prevent intestinal diseases
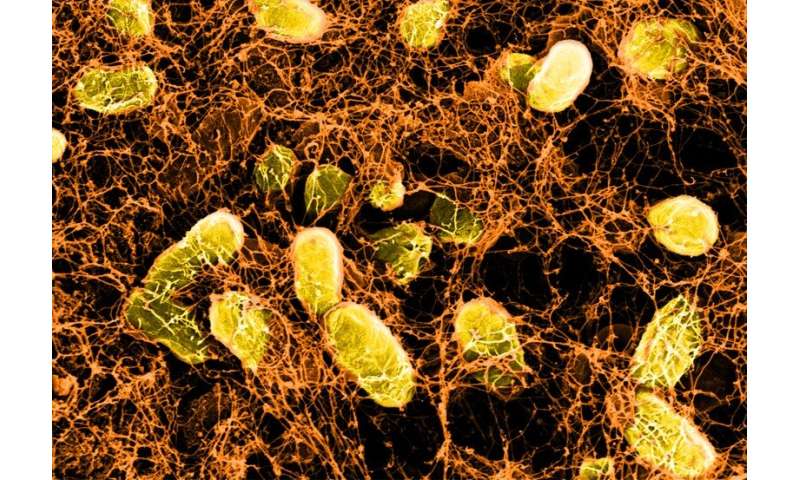
Each one of us carries about 38 trillion bacteria around with us in our gut every day—if you wanted to count them all, it would take you more than a million years. How can such a veritable zoo of microbes reside peacefully in our guts without triggering our immune systems to attack them, as do “bad” bacteria that cause disease? The answer lies in the intestinal mucosal barrier, which includes tightly connected epithelial cells that line the intestine, a layer of dense mucus that protects those cells from bacteria and other gut contents, and immune cells underneath the epithelial cells that quickly kill any microbes that penetrate the barrier.
When the mucosal barrier is disrupted, friendly bacteria become foes as the immune system mounts an inflammatory response against them. This inflammation can impede the mucosal barrier’s healing process, which prolongs the contact between gut microbes and immune cells and creates a vicious feedback loop. Many health problems can occur as a result of this long-term immune response against the gut, including infections, food allergies, diabetes, liver disease, neurological conditions, cancer, and inflammatory bowel disease (IBD), placing an immense burden on patients, nurses, and doctors alike. Scientists at the Wyss Institute are exploring a number of different strategies to overcome this problem and effectively heal the mucosal barrier, which could alleviate the suffering of millions of people around the world.
Immediate immune improvement
IBD is perhaps the most common inflammation-associated intestinal disease, afflicting more than 3 million adults in the United States alone. The principal types of IBD are Crohn’s disease and ulcerative colitis, and the disease can also lead to arthritis, skin disorders, colon cancer, or perforations of the intestines. Because these problems are caused by an overactive immune system, scientific efforts to find a treatment for IBD have started to focus on immune-modulating drugs that can counteract the inflammation response and allow the mucosal barrier to heal. However, most of the compounds that have been identified so far are not fit to be used as medicines, as they carry increased risks of toxicity, infection, and cancer—all side effects of blocking the immune system’s ability to survey the body and defend it.
Elliot Chaikof, M.D., Ph.D., an Associate Faculty member of the Wyss Institute and Surgeon-in-Chief at Beth Israel Deaconess Medical Center, decided to try to solve this problem from a different angle—not by blocking the body’s natural immune defense mechanisms, but by coaxing immune cells to secrete chemicals that promote the repair of the mucosal barrier directly, thus ending the inflammation-causing contact between immune cells and the gut microbiome.
In a recently published paper, Chaikof and his team focused on the protein aryl hydrocarbon receptor (AHR), which is known to change the expression patterns of certain immune-associated genes when activated, and whose deactivation has been shown to cause mucosal barrier dysfunction. The researchers synthesized a collection of compounds whose structures are similar to those that are known to activate AHR, and then used a combination of experiments and computer simulations to identify candidates that showed strong AHR binding.
After multiple rounds of designing and refining the candidate compounds, the team honed in on two, called PY108 and PY109, that seemed the most promising. When they introduced those compounds into human and mouse gut cells, they observed an increase in the amount of AHR activation, and giving them orally to living mice with colitis significantly improved the animals’ health and survival.
The mice treated with PY109 experienced additional positive benefits, including the upregulation of genes coding for interleukins 22 (IL-22) and 17A (IL-17A), which are known to enhance the production of mucus and support barrier cells’ function. When the researchers tested the compound in human T cells derived from patients either with or without IBD, both populations of patient cells displayed an increase in IL-22, suggesting that PY109 could be useful in modulating the immune system—and thus helping heal the mucosal barrier—in humans as well.
“We are now expanding our studies to investigate the effects of AHR-targeting drugs on metabolic syndrome and non-alcoholic fatty liver disease, both of which are also associated with a breach of the intestinal mucosal barrier,” said Chaikof.
A probiotic Band-Aid for your gut
Targeting the immune system to reduce inflammation and allow the gut to heal itself may work for some patients, but those who have compromised immune systems or other problems might not be able to tolerate such treatments. Fortunately, fellow Wyss Institute member Neel Joshi, Ph.D. has created an alternative solution: a probiotic hydrogel that can be applied to the inner surface of the gut like a living bandage to physically seal mucosal barrier breaches and promote healing without involving the immune system.
“Our hydrogel contains a special strain of genetically engineered E. coli bacteria that essentially secretes a biofilm that physically binds to the gut’s mucus layer,” said Joshi, who is a Visiting Scholar at the Wyss Institute and an Associate Professor of Chemistry and Chemical Biology at Northeastern University. “We can easily introduce the hydrogel into the gut using a spray, syringe, endoscope, or oral ingestion, and the gel can regenerate itself because the bacteria within it are constantly producing the adhesive fibers that bind it to the gut surface.”
Joshi’s team described their creation in a recent paper published in Advanced Materials. When they administered their “Live Gels” to mice orally, they found that they survived the stomach’s harsh, acidic environment and remained in the intestine for at least five days—several times longer than existing polymers that aim to increase the persistence of drugs in the intestine. The bacteria residing in the Live Gels can also be killed before the hydrogel is introduced into the gut, for shorter-lived treatments and for patients whose immune systems might attack the benign bacteria in the hydrogels.
The researchers are continuing to refine their approach, dubbed “Probiotic Associated Therapeutic Curli Hybrids” (PATCH), and have demonstrated that it helps reduce inflammation and improve recovery from colitis in mice, which returned to nearly normal function after receiving the treatment orally.
Making the gut do its own gut checks
As with any other disease, early detection of a breach in the gut’s mucosal barrier is key to getting patients the right treatment and maximizing their recovery. However, the symptoms of a disrupted mucosal barrier can easily be mistaken for other, more innocuous conditions like indigestion or gas, and patients are likely to try over-the-counter drugs or at-home remedies rather than see a doctor right away. What if the gut could monitor and treat itself, so that patients wouldn’t have to endure prolonged discomfort and invasive diagnostic tests? That’s the question that Wyss Core Faculty member Pamela Silver, Ph.D. is working to answer by engineering the microbes living in the human gut to detect, report, and treat disease.
Her lab is using synthetic biology techniques to create genetic circuits containing a “trigger element” that senses the presence of a target biomarker and then turns on a “memory element” that records the presence of the target, and can be integrated into strains of bacteria found in the human gut. In a landmark study, Silver’s team inserted a genetic circuit designed to turn on when exposed to tetrathionate (a molecule produced by inflamed intestinal tissue) into E. coli, then administered the bacteria to mice whose guts were infected with pathogenic S. typhimurium. The circuits within the E. coli bacteria flipped to the “on” state when they encountered tetrathionate, and retained the memory of that exposure for up to six months in the mice’s guts.
The researchers are continuing to identify genetic circuits that can sense a wide variety of biomarkers, with the goal of creating even more complex bacteria that can monitor the gut for a host of potential diseases, record the existence of an infection or injury, and even secrete molecules that can heal the mucosal barrier or activate the body’s immune system against a specific infectious microbe.
“These living cellular devices could eventually be developed into a probiotic-like pill containing multiple types of engineered bacteria that can colonize the gut and sense and record several signals at once,” said Silver, who is also the Elliot T. and Onie H. Adams Professor of Biochemistry and Systems Biology at Harvard Medical School (HMS). “This would allow clinicians to essentially ‘fingerprint’ a disease based on the collective signals the bacteria produce, so that they can make an accurate diagnosis non-invasively.”
Bringing the barrier out of the gut and into the lab
All of these methods for healing the gut’s mucosal barrier have so far been validated in mice, but they must all be shown to work in humans before they can be commercialized into therapies. Clinical trials are notoriously expensive and lengthy, and drugs that appear to have a positive effect in mice often show no benefit or are even toxic to humans.
Wyss Institute Founding Director Donald Ingber, M.D., Ph.D. has been working for over a decade on building Organ Chips that can replicate the basic functions of human organs and enable drugs to be tested in a system that more closely mimics a human patient’s body. As part of that project, Ingber’s lab has created a Colon Chip seeded with human intestinal cells that spontaneously produce their own mucus layer and regenerate themselves, just like the living human gut. In their experiments, the mucosal barrier that formed in the chip reacted to a pro-inflammatory molecule by rapidly swelling, indicating that the Colon Chip is a good proxy for studying the effects of inflammation-related gut diseases like IBD. The team also performed a metabolomic analysis inside the Colon Chip and identified specific molecules released by the gut’s microbiome that can actually worsen mucosal barrier damage inflicted by “bad” infectious bacteria.
Source: Read Full Article