Neural responses mediate translation of sustained nociceptive inputs into subjective pain experience
In a dynamic environment, the temporal structures of sensory events evolve at multiple temporal scales, extending from transient to sustained periods. When considering nociceptive stimuli, tracking and predicting the temporal structure of nociceptive inputs is critical to ensure survival, as appropriate and immediate reactions are necessary to avoid actual or potential bodily injury. The brain responses triggered by transient nociceptive stimuli have been extensively studied.
However, the transient brain responses cannot track the temporal structures of sustained nociceptive stimuli, and neural responses triggered by sustained nociceptive stimuli have not fully investigated. Importantly, the neural processes responsible for translating sustained nociceptive information into subjective pain perception are not yet fully elucidated.
Dr. Hu Li and his colleagues from the Institute of Psychology of the Chinese Academy of Sciences, together with researchers from the Istituto Italiano di Tecnologia (Italy), University College London (United Kingdom), and Shenzhen University, investigated the neural correlates underlying the translation of sustained nociceptive inputs into subjective pain experience.
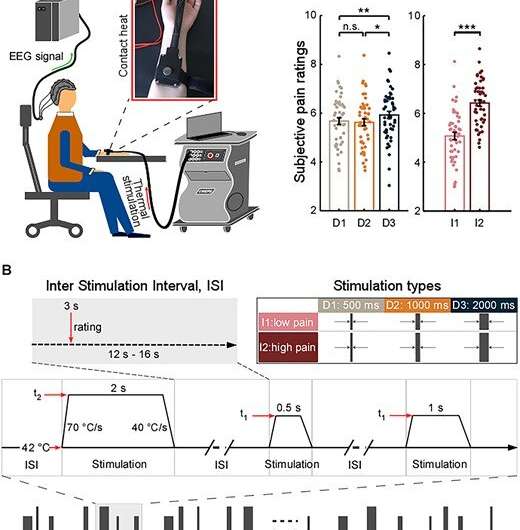
They delivered thermo-nociceptive stimuli with varied durations and intensities on 48 healthy participants. Differences in stimulus processing were quantified by subjective pain ratings and brain activity sampled by EEG.
They found that pain perception and several brain responses are modulated by stimulus duration and intensity.
Crucially, two sustained brain responses related to the emergence of painful percepts were identified: a low-frequency component (LFC, <1Hz) originated from the insula and anterior cingulate cortex, and an alpha-band event-related desynchronization (α-ERD, 8–13 Hz) generated from the sensorimotor cortex. These two sustained brain responses were highly coupled, with the α-oscillation amplitude that fluctuated with the LFC phase.
Furthermore, the translation of stimulus duration into pain perception was serially mediated by α-ERD and LFC.
The study provides novel evidence for ongoing communications between the lateral (e.g., the sensorimotor cortex) and medial (e.g., the insula and anterior cingulate cortex) pain systems, thus shedding new light on the importance of the interplay of neural responses at different frequencies in different brain regions for the generation of pain perception.
The findings might be used in the future for exploring clinical pain, given that sustained nociceptive stimuli more effectively simulate the dynamics of spontaneous pain in clinical conditions.
Source: Read Full Article