Novel biomarker for immune checkpoint inhibitors identified in human lethal cancers
A new study, published online ahead of print in Journal for ImmunoTherapy of Cancer on December 6, has identified a novel biomarker for ICIs in human cancers.
Immune checkpoint inhibitors (ICIs) mainly include antibodies targeting proteins such as the programmed cell death protein-1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). ICIs immunotherapies have revolutionized the treatment of many lethal cancers. However, only a minority (approximately 20%) of patients benefit from ICIs immunotherapy. It is therefore important to identify patients who are more likely to benefit from ICIs.
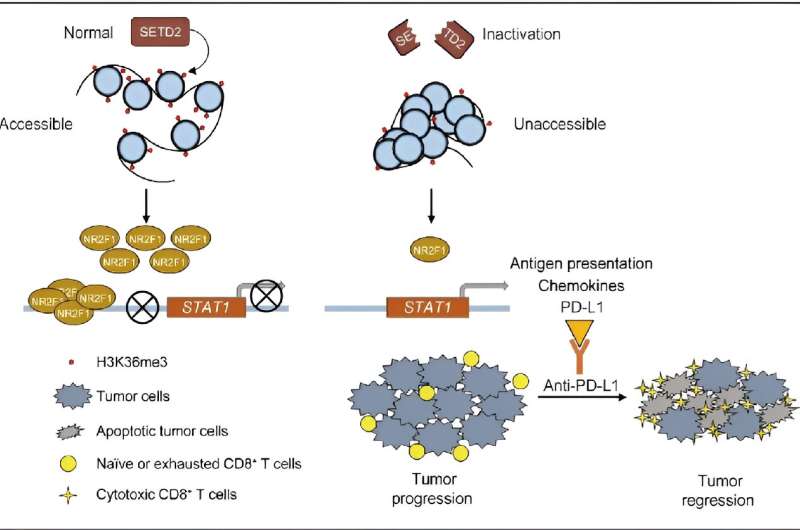
SETD2 is the only known histone methyltransferase responsible for the trimethylation of histone H3 at lysine 36 (H3K36me3), playing a critical role in gene regulation through H3K36me3. Genomic mutations of SETD2 can be found in ~ 5% of human cancers and they promote tumor progression and metastasis in conditions such as clear cell renal cell carcinoma, pancreatic cancer, colorectal cancer, prostate cancer and breast cancer. However, no small molecules or antibodies that specifically target SETD2-deficient tumors and show therapeutic potential have been developed.
In exploring the relationship between the SETD2 gene and cancer immune therapy, a research team led by Prof. Wang Yuexiang from Shanghai Institute of Nutrition and Health of the Chinese Academy of Sciences analyzed a queue containing 1,662 patients treated with ICIs.
Their prognosis and omics datasets identified 127 genes correlated with a positive response to immunotherapy. In addition to established biomarkers for ICIs therapy (e.g., TP53, PAK7, TET1, PTPRD/PTPRT and STK1), SETD2 ranked among the top three genes. Patients with SETD2 inactivating mutations showed more favorable responses to immunotherapy.
Functional experiments proved that murine syngeneic tumors harboring Setd2 inactivation were sensitive to ICIs. By bulk and single-cell RNA-seq, the research team further revealed that SETD2 inactivation reprograms intratumoral immune cells and inflames the tumor microenvironment.
Mechanistically, an integrated multiomics analysis using ATAC-seq, ChIP-seq and RNA-seq demonstrated that SETD2 inactivation reduces NR2F1 transcription by impairing H3K36me3 deposition and chromatin accessibility. This in turn activates the STAT1 signaling pathway, which enhances the expression of chemokines and PD-L1, thereby improving antigen presentation.
The findings of this study provide novel insights into the biology of SETD2 inactivation regulation and reveal a novel biomarker for identifying cancer patients who may benefit from ICI therapy, particularly for those with cancers such as pancreatic cancer, melanoma and renal cancer.
More information:
Xufen Zheng et al, Tumor cell-intrinsic SETD2 inactivation sensitizes cancer cells to immune checkpoint blockade through the NR2F1-STAT1 pathway, Journal for ImmunoTherapy of Cancer (2023). DOI: 10.1136/jitc-2023-007678
Journal information:
Journal for ImmunoTherapy of Cancer
Source: Read Full Article