An Experimental Drug Protects Covid-19 Patients, Eli Lilly Claims
- In a reversal, the Big Ten Conference will try to play football in 2020.
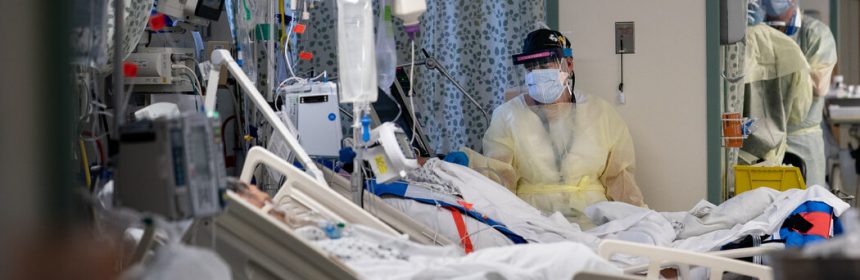
- An experimental drug is showing promise in reducing blood levels of the virus for newly infected patients.
- The C.D.C. director is tasked with defending what many see as a hobbled agency before a Senate panel.
“It’s exciting,” said Dr. Cohen, who was not involved in the study. The clinical trial appears to be rigorous, and the results are “really compelling.”
Other companies, too, are developing monoclonal antibodies to combat the coronavirus, he noted: “This is the opening of a door.”
The study will eventually enroll 800 patients of all ages and in all risk categories at sites across the United States. So far the research has proceeded with unusual speed.
In six months, Eli Lilly isolated an antibody from one of the first Covid-19 survivors, turned it into a drug and began a study, enrolling the first patients on June 17. “It was an all-out effort,” said Dr. Daniel Skovronsky, the company’s chief scientific officer.
The goal was to enroll patients as soon as they were infected, but that meant accelerating the usual selection process. Trial sites administered rapid diagnostic tests and moved quickly to enroll eligible patients.
Eli Lilly is also testing the drug in nursing homes to see if it prevents infections in residents and staff members.
In addition to constantly measuring the amount of the coronavirus in blood, the investigators also sought to understand whether the pathogen was mutating to avoid antibodies.
They found that the virus was changing, to some extent: 8 percent of the viruses had mutated in participants getting the drug, compared with 6 percent in those receiving the placebo. (Presumably, the virus also was trying to dodge the natural antibodies that placebo patients were making on their own.)
Coronavirus Drug and Treatment Tracker
An updated list of potential treatments for Covid-19.
The investigators expected that their drug might produce a reduction in the amount of virus in patients’ blood. They did not anticipate a sharp reduction in patients who needed hospitalization.
“This is the first time we have ever seen anything of this magnitude,” Dr. Skovronsky said.
The antibody drug did not produce significant side effects, he said. Patients received a single infusion, providing antibodies that should last about a month.
There is good news regarding a vaccine in these findings. If monoclonal antibodies had not worked, then the finding may have cast doubt on the notion that the virus can be stopped with antibodies.
On the other hand, the results — if they are proven accurate — do not guarantee that a vaccine will work. Eli Lilly’s monoclonal antibody is a temporary treatment; a vaccine is designed to elicit long-lasting natural antibodies and thus immunity.
Like other companies, Eli Lilly has been manufacturing large quantities of its drug — 100,000 doses — in hopes that it turns out to be effective.
The company will be discussing its data with the Food and Drug Administration, Dr. Skovronsky said, along with the possibility of obtaining an emergency use authorization allowing Eli Lilly to market the drug.
Monoclonal antibodies are expensive to make and carry high price tags, often thousands of dollars per dose. But if the findings hold up, there will be comfort for the public in knowing there is something doctors can do to head off dire illness, Dr. Cohen said.
“For my wife and I, who are older and fatter — we are waiting for drugs like this so we can see our grandchildren,” he added.
Site Index
Site Information Navigation
Source: Read Full Article
